
eBook - ePub
The Wiley-Blackwell Handbook of Psychoneuroimmunology
Alexander W. Kusnecov, Hymie Anisman, Alexander W. Kusnecov, Hymie Anisman
This is a test
Share book
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
The Wiley-Blackwell Handbook of Psychoneuroimmunology
Alexander W. Kusnecov, Hymie Anisman, Alexander W. Kusnecov, Hymie Anisman
Book details
Book preview
Table of contents
Citations
About This Book
This comprehensive resource details the history, methodology and development of research into psychoneuroimmunology, balancing it with meticulous coverage of both the clinical aspects and practical applications of the subject.
- A much-needed reference including overviews of key advances in the field
- Discusses how psychoneuroimmunological research is conceived and executed
- Includes contributions from a wealth of experts in the field
- Forward by Robert Ader and Nicholas Cohen, founders of the discipline
- Authoritative and interdisciplinary in scope - integrating biological and behavioral science
Frequently asked questions
How do I cancel my subscription?
Can/how do I download books?
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
What is the difference between the pricing plans?
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
What is Perlego?
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Do you support text-to-speech?
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Is The Wiley-Blackwell Handbook of Psychoneuroimmunology an online PDF/ePUB?
Yes, you can access The Wiley-Blackwell Handbook of Psychoneuroimmunology by Alexander W. Kusnecov, Hymie Anisman, Alexander W. Kusnecov, Hymie Anisman in PDF and/or ePUB format, as well as other popular books in Psychology & Neuropsychology. We have over one million books available in our catalogue for you to explore.
Information
1
Basic Principles in Immunology
Relevance for Studies in Psychoneuroimmunology
Introduction
Over the past century our knowledge of the immune system and how it functions has grown exponentially. This is especially true in regard to how it relates to and interacts with various physiological systems, including the central nervous system. An important focus of the field of neuroimmunology is to elucidate the ways that the immune system influences neuronal function and subsequently, behavior and cognition through the modulation of cytokines and hormones, especially stress hormones such as corticosteroids. Since the intimate relationship between the immune system and brain function has come to light, research in this field has broadened into psychoneuroimmunology, which specifically addresses the role of the immune system in the development of psychiatric disorders, including depression and anxiety. The purpose of this section is to provide a general overview of basic immune function, describing both the components of the immune system and the various modes of immunity employed in an immune response. Additionally, we will explore the validity of some of the most widely used methods and models for psychoneuroimmunology applied to the study of interactions between immunological processes and behavior and cognition as they relate to mental disorders in humans.
The Components of the Immune System
To understand how the immune system influences the brain, and subsequently, behavior and cognition, it is vital to understand how the immune system functions. The immune system comprises two major components: specialized cells that carry out the various functions of the immune process, and the chemical messengers that allow these cells to communicate, not only with each other, but with other cells and tissues within the body. These partners in immune function must perform a precise and complex dance in order to maintain homeostasis and, when necessary, to mediate an inflammatory response. In general, as part of the inflammatory response damaged or infected cells secrete chemical messengers called chemokines that serve to attract specific immune cells, which in turn release various cytokines that influence the types of cells and modes of immunity that will be employed to eliminate any potential pathogens. Once these threats have been neutralized the process continues, as immune cells and their chemical messengers also function to mediate tissue repair and regeneration. A lack of coordination from either partner can result in deleterious consequences, including the development of allergies, as well as autoimmune and immune-deficiency disorders.
Cytokines and chemokines – the immune system's messengers
Cytokines and chemokines are protein and glycoprotein molecules synthesized and secreted by cells as part of the immune response. Chemokines are a specialized class of cytokines that derive their name from their role in chemotaxis; a majority of these soluble factors are chemoattractants that serve to guide immune cells to the site of infection. They are characterized by their small size and the presence of four cysteine residues (named C) which contribute to their tertiary structure. They are divided into four families (C, CC, CXC and CX3C) based upon the location of the first two C residues. Chemokines in the C group differ from the other chemokine families in that they contain only two cysteines; secretion of these chemokines attracts T-cell progenitors to the thymus. The CC chemokines have two adjacent cysteines near the amino terminus, while the relevant cysteines in the CXC chemokines can be found at the N-terminus separated by a single amino acid (X). Similarly, the CX3C chemokines have three intervening amino acids; thus far, fractalkine is the only chemokine with this structure that has been identified. In addition to their role in immune function, chemokines contribute to a variety of biological functions, especially in the brain, as will be discussed later.
The major cytokines consist of interleukins (IL), interferons (IFN) and colony-stimulating factors, as well as various growth factors and eicosanoids, including prostaglandins. Cytokines are mainly produced by immune cells and also by a variety of other cell types including brain cells. The specificity of the elicited immune response is dictated by the expression of cytokine receptors that are widely expressed in tissues and organs. Additionally, some cytokine receptors exist in a soluble form and can act as inhibitors of cytokine activity through competitive binding of their ligands. To differentiate between cytokines' biological activity they are often described as either pro-inflammatory or anti-inflammatory; upon damage to or infection of cells and tissues, pro-inflammatory cytokines are produced and secreted to stimulate immune system activation. The induction of cytokine expression tends to occur in a step-wise manner, with the expression of certain cytokines dependent upon the prior expression of others; for example, IL-1 is necessary to induce the production of IL-2, IL-6 and tumor necrosis factor (TNF). Anti-inflammatory cytokines, such as IL-10, are also released during inflammation in order to dampen and eventually terminate pro-inflammatory cytokine activity. In many instances, the cytokines IL-4 and IL-13 are referred as anti-inflammatory because they oppose the effects of inflammatory cytokines IL-2 and IFN-γ. However, many inflammatory processes such as allergic inflammation are mediated by the actions of IL-4 and IL-13. Thus, describing cytokines purely by their pro- or anti-inflammatory properties can be misleading since they are pleiotropic in nature and are involved in many biological processes. Maintaining a balance in cytokine and chemokine signaling is vital for sustaining immune homeostasis and stimulating the appropriate immune cells as part of the immune response.
The cells of the immune system
The circulatory system serves as the main highway for the cells of the immune system, so it is not surprising that immune cells are derived from the same source as the other major components of blood. During prenatal development the spleen and liver are responsible for producing both red blood cells and white blood cells; however, once the skeleton begins to develop and the bone marrow becomes established, this responsibility shifts to hematopoietic stem cells (HSCs) within the bone marrow. HSCs give rise to the three cell lineages of the blood and immune system: the erythroid lineage, the myeloid lineage, and the lymphoid lineage (see Figure 1.1). Currently, the general consensus for how these lineages arise is that the initial progeny of HSCs are multipotent progenitor cells (MPPs) which in turn give rise to common myeloid progenitor cells (CMPs). Progeny of these CMPs maintain expression of myeloid specific genes, but can undergo further restriction into either erythroid or lymphoid progenitors. Thus, the myeloid lineage may be considered the default fate for CMPs unless directed towards either erythroid or lymphoid lineages through changes within the milieu of the stem cell niche, including alterations in cytokine expression. Ultimately, the erythroid lineage will develop into red blood cells and platelets while the myeloid and lymphoid lineages will give rise to the cells of the immune system.
Figure 1.1 Cells of the immune system. Hematopoietic stem cells (HSCs) within the bone marrow are relatively quiescent stem cells whose progeny, multipotent progenitor cells (MPPs), can differentiate into both erythrocytes and leukocytes. There are three potential lineage fates: the erythroid lineage, which gives rise to both red blood cells (RBCs) and platelets, and the myeloid and the lymphoid lineages, which produce the cells of the immune system. Myeloid progenitor cells differentiate within the bone marrow to produce monocytes, granulocytes and mast cells, which then migrate to their target environments within the blood and tissue. Lymphoid progenitors can also be found within the bone marrow, however these cells will differentiate into precursor B-cells that will then migrate into the lymphatic tissues and organs. In contrast, T-cell progenitors leave the bone marrow and migrate directly to the thymus where they will undergo further proliferation and selection for immunocompetency.
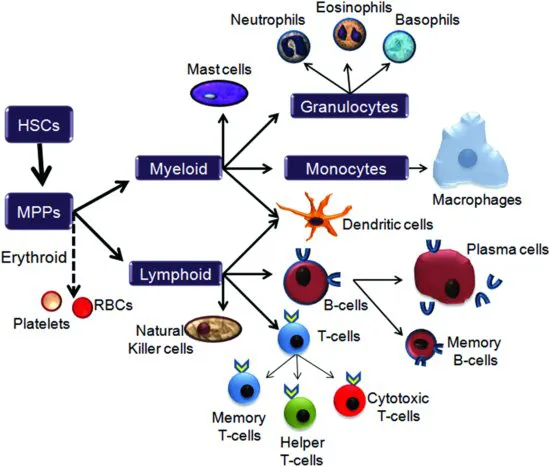
The myeloid lineage
Members of the myeloid lineage include monocytes, granulocytes and mast cells. The primary function of monocytes is to migrate out of the vasculature and into tissues where they mature into macrophages that will monitor the body and destroy potential pathogens through phagocytosis. Macrophages can be further classified into mobile or fixed macrophages. The alveolar macrophages of the lungs and the dendritic cells of the epidermis are examples of mobile macrophages that can freely travel within the interstitial space, whereas the Kupffer cells of the liver remain fixed in place.
The granulocytes, named for the multiple granules found within their cells, comprise three types of cells: neutrophils, eosinophils, and basophils. These polymorphonuclear cells (PMNs) are confined primarily to the blood stream until activation by cytokines and chemokines released by damaged cells and tissues. These messengers prompt the PMNs to migrate into the interstitial space where they will hunt down and destroy invading pathogens. Neutrophils, which make up the greatest proportion of PMNs, are phagocytic cells that are among the first cells recruited to eliminate invading pathogens. In addition to destroying foreign cells by phagocytosis, neutrophils can also degranulate and release anti-microbial chemicals such as gelatinase and cathepsin. Interestingly, neutrophils have also been observed extruding filaments of DNA and associated proteins that can act as nets to entrap microbes; these extracellular structures provide an alternate method of destroying pathogens and may prevent their spread into the surrounding tissue. Lastly, neutrophils also release cytokines and thus can enhance the inflammatory response by recruiting more immune cells to the site of infection. The other two types of granulocytes, the eosinophils and basophils, make up a relatively small proportion of the total leukocyte population, but are vital in mitigating the effects of pathogens, especially for their role in mediating the innate and adaptive immune responses. Although eosinophils and basophils are perhaps best characterized for their anti-parasitical activities, in recent years their role in tissue and immune homeostasis has been further clarified. As part of the adaptive immune response, eosinophils are rapidly recruited to the site of infection by T-helper 2 (TH2) cells, where they release cytokines and lipid mediators, such as prostaglandin 2, as well as cytotoxic chemicals that can destroy invading pathogens. Additionally, they have the capability of acting as antigen-presenting cells to activate both naïve and memory T-cells. Finally, both eosinophils and basophils have also been implicated in the development of hypersensitivity and allergies, perhaps due to their relationship with TH2 cells and mast cells.
Mast cells are functionally and morphologically similar to eosinophils and basophils; they play a vital role in the immunity against parasites, and facilitate tissue repair by stimulating angiogenesis, the growth of new blood vessels. However, these myeloid cells are found mainly within tissues adjacent to the external environment, especially within the mucosae of the respiratory and gastrointestinal tracts, and are perhaps best-known for their role in allergic responses. Mast cells are also found in the brain, particularly in some nuclei of the thalamus. The granules within mast cells store a variety of cytokines and chemokines that facilitate the inflammatory process, as well as histamine which not only dilates blood vessels and is responsible for the pain and itchiness associated with an allergic reaction, but which can also act as a neurotransmitter. Another neurotransmitter, serotonin, has also been found within mast cells, and although the role of these neurotransmitters is not yet clear, they may be involved in cross talk between the immune system and neurons, especially those of the enteric nervous system. Interestingly, in addition to direct damage or the binding of antigens, degranulation of mast cells can also be initiated by various neuropeptides, further supporting the possibility that mast cells represent a link between the immune system and the nervous system.
The lymphoid lineage
Lymphocytes derive their name from the fact that they reside primarily within the tissues of the lymphatic system. These tissues include a network of reticular fibers that can be found in virtually every organ of the body; these fibers converge upon the lymph nodes and the two major organs of the lymphatic system: the spleen and the thymus. The main function of the lymph nodes is to filter out and clear lymph as it travels along the lymphatic vessels. Resident macrophages remove and destroy any microbes or cellular debris while lymphocytes monitor the lymphatic stream for the presence of foreign antigens. The lymphocytes include B-lymphocytes, T-lymphocytes and natural killer (NK) cells.
B-cells differentiate within the bone marrow and migrate into the lymph nodes and spleen. Here they will remain in a precursor stage until activated by an antigen, at which time they will undergo rapid proliferation and maturation into antibody-secreting plasma cells. Membrane-bound immunoglobulins (Ig), including IgM and IgD, on the surface of precursor B-cells act as receptors for intact antigens. The binding of the antigen stimulates the production of secretory immunoglobulins, usually referred to as antibodies, including IgM, IgG, IgA and IgE. These antibodies consist of a conserved region and a variable region. It is the conformation of the variable region (the product of the genetic recombination of several genes within the immunoglobulin super-gene family) that makes the antibodies specific for their target antigen. Interestingly, lymphocytes are the only somatic cells that rearrange DNA to produce new protein variants as part of their phenotype.
Once antibodies have been secreted into the extracellular space they can facilitate the removal of pathogens in a variety of ways. By binding to antigens on the surface of pathogens they can make the pathogen more visible to macrophages. That is, the antibody serves as an opsonin (from the latin “to relish”), that marks the pathogen as a target for phagocytosis by macrophages; this will be facilitated by the Fc region of the antibody molecule binding to Fc receptors on the macrophage. Additionally, some immunoglobulins are capable of binding to and activating other effector cells, including granulocytes and mast cells. In the case of IgG, binding to platelets allows for the transfer of immunity across the placenta, which is vital for the development of the fetal immune system. The binding of the Fc region of IgE to the Fc receptor on mast cells results in mast cell degranulation and release of inflammatory mediators such as histamine. The production of IgE antibodies against harmless compounds such as pollen or albumin is responsible for the establishment of allergies.
Although the role of different antibodies in the immune response is quite varied, their primary function is to facilitate the removal of pathoge...