
eBook - ePub
Biotechnology and Biopharmaceuticals
Transforming Proteins and Genes into Drugs
This is a test
Share book
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Biotechnology and Biopharmaceuticals
Transforming Proteins and Genes into Drugs
Book details
Book preview
Table of contents
Citations
About This Book
Biotechnology and Biopharmaceuticals: Transforming Proteins and Genes into Drugs, Second Edition addresses the pivotal issues relating to translational science, including preclinical and clinical drug development, regulatory science, pharmaco-economics and cost-effectiveness considerations. The new edition also provides an update on new proteins and genetic medicines, the translational and integrated sciences that continue to fuel the innovations in medicine, as well as the new areas of therapeutic development including cancer vaccines, stem cell therapeutics, and cell-based therapies.
Frequently asked questions
How do I cancel my subscription?
Can/how do I download books?
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
What is the difference between the pricing plans?
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
What is Perlego?
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Do you support text-to-speech?
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Is Biotechnology and Biopharmaceuticals an online PDF/ePUB?
Yes, you can access Biotechnology and Biopharmaceuticals by in PDF and/or ePUB format, as well as other popular books in Biological Sciences & Biotechnology. We have over one million books available in our catalogue for you to explore.
Information
Part I
TRANSFORMING PROTEINS AND GENES INTO DRUGS
The Science and the Art
Transformation of biotechnology and scientific discoveries into therapeutic products is a science and could be considered as an art. Biopharmaceutical products are mainly derived from peptides, nucleic acid polymers, and proteins. They are often referred to as biologics, biomolecules, biotherapeutics, macromolecules, and protein therapeutics. In this book, we will use these terms interchangeably with the term biopharmaceuticals. The following eight chapters introduce the process of leveraging a series of discoveries in biological macromolecules, including identification of their structures and elucidating their physiological roles for application as therapeutic agents. The path to prove a therapeutic molecule is safe and effective for patients is often complex and depends on science and more. With the advancement of recombinant DNA technology and the rapid growth in automation efficiency and computing power, many more drug targets are available to produce (recombinant or synthetic) drugs or pharmaceuticals. The discussions that follow document the knowledge and the experience gained from transforming biological macromolecules into drugs. The series of chapters highlight some of the key differences between the discovery and development of small-molecule drugs and high-molecular-weight biopharmaceuticals. The growing appreciation of the pivotal roles that public and private organizations play in supporting the enabling technologies through financial resources and leveraging all resources for drug development is an evolving art. Transformation of biomolecules into biopharmaceuticals includes building capacity, evaluating therapeutic potential, and testing whether a molecule clears a defined set of tests in a test tube, in animals, and eventually in humans. The therapeutic molecules that clear the safety and efficacy evaluations and become endorsed by the US Food and Drug Administration are approved for human use. How the drug industry develops a price for a new therapy is an art. These integrated topics have proved to be increasingly important for decision makers, pharmaceutical scientists, and physicians in their practice.
1
INTRODUCTION TO BIOPHARMACEUTICALS
1.1. Background and Significance
1.2. Translation of Biotechnology for Developing Biopharmaceuticals
1.3. Historical Perspective of Pharmaceutical Biotechnology
1.4. Distinctions between Chemical Drugs Versus Biopharmaceuticals
1.5. Summary
Biopharmaceuticals, otherwise known as the application of biomolecules as therapeutic products, have benefited from advances in the study of biology and biological interactions of simple and complex organisms including prokaryotes, eukaryotes, and mammalian systems. Basic discoveries and a greater understanding of biochemistry and biophysics have shed light on the abnormalities of the highly coordinated biological systems in humans that are related to disease symptoms. These discoveries have allowed for innovations to be made in the design and development of biopharmaceuticals for treating a wide range of human diseases. While biotechnology today is synonymous with advanced technologies, the technology of using biological molecules as therapeutics has been in existence since the 1800 s. Ever since elucidating that the human body is composed of specialized cells and proteins, exponential advances have provided enabling technologies that consistently produce high-quality proteins, antibodies, and peptides for pharmaceutical applications. Continued refinement and optimization of the production of recombinant macromolecules—enzymes, growth hormones, vaccines, and monoclonal antibodies—have fueled, and will continue to fuel, the growth and influence in overall drug development. When this text was first published in 2003, only a handful of biopharmaceuticals reached US $1 billion in annual sales. At the time of writing this second edition, the top-selling biopharmaceuticals reached US $7.3 billion, and the top 25 biopharmaceutical products generated US $74.7 billion in 2010. With over 200 biopharmaceutical products on the market, these achievements were possible because of the outstanding contribution of scientists and clinicians and their collective efforts to collaborate and integrate innovations into novel therapeutic products. This chapter defines the differences between small-molecule or traditional drugs and biologics or biotherapeutics—proteins, peptides, and biological materials—that are much larger molecules. A small change at the atomic level for a small-molecule drug typically leads to a new drug with a unique set of therapeutic and side effects, whereas a modification of amino acids (with multiple atomic modifications) on protein-based biotherapeutics, such as insulin and hepatitis B vaccine, retains a very similar therapeutic profile and clinical application. This chapter introduces in an easy-to-read level the growth in new biopharmaceuticals reaching the market, their therapeutic importance, and their overall contribution to health care. It is intended for students, health professionals, legislators, decision makers, and pharmaceutical researchers who want to learn about the science and business of biotechnology and its role in transforming biological discoveries into therapeutic products.
1.1. BACKGROUND AND SIGNIFICANCE
For most people, biotechnology is synonymous with “high-technology or advanced technology”. However, the idea to use technology or products derived from biological molecules and processes for disease treatment is not new. Even before the discovery that the human body is composed of cells and proteins, humans were constantly being challenged by invading pathogenic microbes and other deadly infections. These real and perceived battlefields of disease necessitated innovations for developing curative medicines—biologically active therapeutic products now recognized as biopharmaceuticals. While biotechnology today is seen as the cutting edge of life sciences, the use of biological molecules as therapeutic agents or biologics has existed since the 1800 s. In fact, the word biotechnology can be traced back to the 1919 writing of Kark Erely in his 84-page publication entitled, “Biotechnologie der Fleisch-, Fett- und Milcherzeugung im landwirtschafttichen Gross-betrieb” (Bud 1989). The coining of the term biotechnologie or “biotechnology” by Erely was likely intended to describe the interaction of biology with technology, thus essentially implying inclusion of all biological and related technologies in product transformation. Today, the therapeutic products of biotechnologies, which are referred to as biologics or biopharmaceuticals, are central in providing hope and in making advances for treating human diseases ranging from infections, diabetes, and immune disorders to cancers. Biopharmaceuticals are derived from peptides and proteins, which are often referred to as biologics, biomolecules, biotherapeutics, macromolecules, and protein therapeutics. In this book, we will use these terms interchangeably when referring to biopharmaceuticals.
Figure 1.1. Time progression of milestones and overall impact on translation of biological molecules into therapeutic products. The discovery of protein, cell, bacteria, and Mendelian genetics in 1830–1900, and the innovative milestones in modern genetics and molecular engineering, provided the basis for exponential growth in the ability to identify, validate, and produce biological molecules for therapeutic applications. The accumulation and expansion of impact is represented on the x-axis. For color detail, please see color plate section.
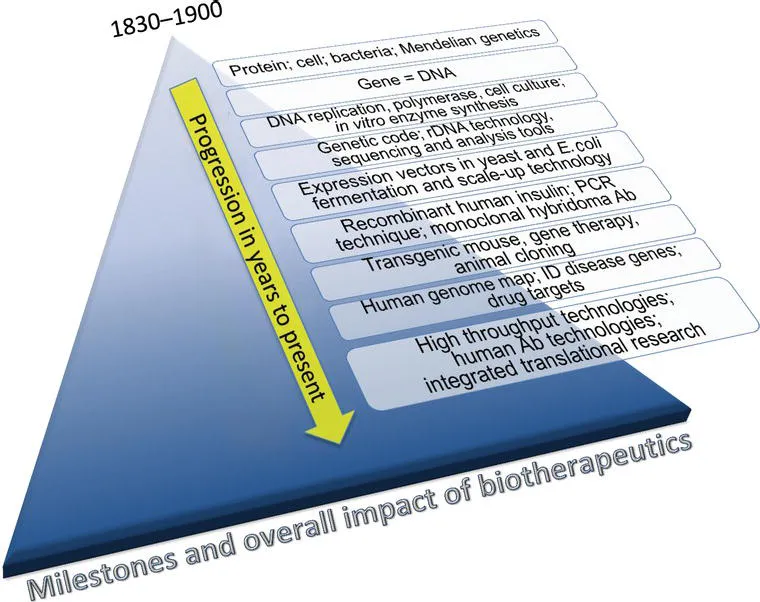
The transformation of basic biological processes and endogenous proteins to biopharmaceuticals that treat disease and provide cures requires integration of scientific discovery and ingenuity into product development. The synthesis of biopharmaceuticals—proteins, peptides, and genetic materials— at a quality and quantity suitable for therapeutic use is a recent achievement. Some of the milestones and innovations pivotal to therapeutic achievements are highlighted in Figure 1.1. Clearly, basic knowledge about the DNA and the genetic code, different cells that make up tissues and organs, and protein synthesis and cellular mechanisms provided the foundation for exponential growth in biotechnology. Some of the significant biotechnology milestones and innovations are (1) recombinant DNA technology (procedures that join together, or recombine, DNA segments) to produce human protein in foreign host cells (Cohen, Chang et al. 1973); (2) cell and fermentation technologies for large-scale protein production (Goeddel 1990); and (3) monoclonal antibody technologies (Kohler and Milstein 1975) that provide antibody therapeutics for treating immune or other disorders and cancers. These technological milestones have enabled transformation of biomolecules into biotherapeutics, which now impact health every day. Without transformational biotechnologies, the health impacts of biotherapeutics such as proteins, antibodies, and enzymes (some of which are still available as tissue- or plasma-extracted products), would have been realized much later. Figure 1.1 also highlights the integration and potential impact due to the ever-expanding knowledge of biological processes and bioengineering. These scientific and engineering achievements have allowed development and use of protein- and antibody-based therapies that require large doses (typically in milligrams or higher amounts) to impact patient health.
Translating biotechnology innovations into therapeutic products requires investment by biopharmaceutical companies that focus on preclinical and clinical product development. While there are many entrepreneurial biotechnology start-up companies working on early-stage therapeutics, a majority of pioneering biotechnology companies, such as Genentech, Chiron, Cetus, and Immunex, that had success in developing therapeutic products, are eventually acquired by large pharmaceutical companies. This strategic acquisition of biotechnology companies has accelerated over the past 10 years. As a result, as shown in Table 1.1, Amgen is the only independent biotechnology company on the list. The other two companies, Genzyme and Biogen Idec, are part of or in the process of being integrated into large pharmaceutical companies. Table 1.1 also compares biotechnology and integrated biopharmaceutical companies and their 2010 revenue, market share, productivity as measured by revenue per employee, and investment in research and development (R&D). Although the total employee numbers are still relatively small, all of the listed biotechnology companies have grown to realize multi-billion dollar annual revenues, and their productivity is comparable to that of integrated biopharmaceutical companies ($632,000 vs. $506,000 per employee, respectively; Table 1.1). Biotechnology companies spend more than 20% (mean = 26%) of their revenue on R&D. This is well above the 11%–24% (mean = 16%) of revenue inve...