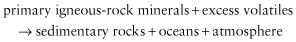![]()
1
Introduction
The fundamental question underlying marine geochemistry is, ‘How do the oceans work as a chemical system?’ At present, that question cannot be answered fully. The past four decades or so, however, have seen a number of ‘quantum leaps’ in our understanding of some aspects of marine geochemistry. Three principal factors have made these leaps possible:
1 advances in sampling and analytical techniques;
2 the development of theoretical concepts;
3 the setting up of large-scale international oceanographic programmes (e.g. DSDP, MANOP, HEBBLE, GEOSECS, TTO, VERTEX, JGOFS, SEAREX, WOCE), which have extended the marine geochemistry database to a global ocean scale.
1.1 Setting the Background: a Unified ‘Process-Orientated’ Approach to Marine Geochemistry
Oceanography attracts scientists from a variety of disciplines, including chemistry, geology, physics, biology and meteorology. A knowledge of at least some aspects of marine geochemistry is an essential requirement for scientists from all these disciplines and for students who take courses in oceanography at any level. The present volume has been written, therefore, with the aim of bringing together the recent advances in marine geochemistry in a form that can be understood by all those scientists who use the oceans as a natural laboratory and not just by marine chemists themselves. Furthermore, the oceans are a key component of the Earth System, so an understanding of ocean geochemistry is central to understanding the functioning of the Earth as an integrated system (Lenton and Watson, 2011). One of the major challenges involved in doing this, however, is to provide a coherent global ocean framework within which marine geochemistry can be described in a manner that cannot only relate readily to the other oceanographic disciplines but also can accommodate future advances in the subject. To develop such a framework, it is necessary to explore some of the basic concepts that underlie marine geochemistry.
Geochemical balance calculations show that a number of elements that could not have come from the weathering of igneous rocks are present at the Earth’s surface. It is now generally accepted that these elements, which are termed the excess volatiles, have originated from the degassing of the Earth’s interior. The excess volatiles, which include H and O (combined as H2O), C, Cl, N, S, B, Br and F, are especially abundant in the atmosphere and the oceans. It is believed, therefore, that both the atmosphere and the oceans were generated by the degassing of the Earth’s interior. In terms of global cycling, Mackenzie (1975) suggested that sedimentary rocks are the product of a long-term titration of primary igneous-rock minerals by acids associated with the excess volatiles, a process that can be expressed as:
As this reaction proceeds, the seawater reservoir is continuously subjected to material fluxes, which are delivered along various pathways from external sources. The oceans therefore are a flux-dominated system. Seawater, however, is not a static reservoir in which the material has simply accumulated over geological time, otherwise it would have a very different composition from that which it has at present; for example, the material supplied over geological time far exceeds the amount now present in seawater. Further, the composition of seawater appears not to have changed markedly over very long periods of time; at least the last few million years and probably longer. Rather than acting as an accumulator, therefore, the flux-dominated seawater reservoir can be regarded as a reactor. Elements are intensively recycled within the vast oceans by biological and chemical processes, although the extent of this recycling and the associated lifetime of components of the chemical system within the oceans vary enormously. It is the nature of the reactions that take place within the reservoir, that is the manner in which it responds to the material fluxes, which defines the composition of seawater via an input → internal reactivity → output cycle. The system is ultimately balanced by the Earth’s geological tectonic cycle that subducts ocean sediments into the Earth’s interior and returns them to the land surface.
Traditionally, there have been two schools of thought on the overall nature of the processes that operate to control the composition of seawater.
1 In the equilibrium ocean concept, a state of chemical equilibrium is presumed to exist between seawater and sediments via reactions that are reversible in nature. Thus, if the supply of dissolved elements to seawater were to increase, or decrease, the equilibrium reactions would change in the appropriate direction to accommodate the fluctuations.
2 In the alternative steady-state ocean concept, it is assumed that the input of material to the system is balanced by its output, that is, the reactions involved proceed in one direction only. In this type of ocean, fluctuations in input magnitudes would simply result in changes in the rates of the removal reactions, and the concentrations of the reactants in seawater would be maintained.
At present, the generally held view supports the steady-state ocean concept. Whichever theory is accepted, however, it is apparent that the oceans must be treated as a unified input–output type of system, in which materials stored in the seawater, the sediment and the rock reservoirs interact, sometimes via recycling stages, to control the composition of seawater.
It is clear, therefore, that the first requirement necessary to address the question ‘How do the oceans work as a chemical system?’ is to treat the seawater, sediment and rock reservoirs as a unified system. It is also apparent that one of the keys to solving the question lies in understanding the nature of the chemical, geological and biological (biogeochemical) processes that control the composition of seawater and how these interact with the physical transport within the ocean system, as this is the reservoir through which the material fluxes flow in the input → internal reactivity → output cycle. In order to provide a unified ocean framework within which to describe the recent advances in marine geochemistry in terms of this cycle, it is therefore necessary to understand the nature and magnitude of the fluxes that deliver material to the oceans (the input stage), the reactive processes associated with the throughput of the material through the seawater reservoir (the internal reactivity stage), and the nature and magnitude of the fluxes that take the material out of seawater into the sinks (the output stage).
The material that flows through the system includes inorganic and organic components in both dissolved and particulate forms, and a wide variety of these components will be described in the text. In order to avoid falling into the trap of not being able to see the wood for the trees in the morass of data, however, it is essential to recognize the importance of the processes that affect constituents in the source-to-sink cycle. Rather than taking an element-by-element ‘periodic table’ approach to marine geochemistry, the treatment adopted in the present volume will involve a process-orientated approach, in which the emphasis will be placed on identifying the key processes that operate within the cycle. The treatment will include both natural and anthropogenic materials, but it is not the intention to offer a specialized overview of marine pollution. This treatment does not in any way underrate the importance of marine pollution. Rather, it is directed towards the concept that it is necessary first to understand the natural processes that control the chemistry of the ocean system, because it is largely these same processes that affect the cycles of the anthropogenic constituents.
Since the oceans were first formed, sediments have stored material, and thus have recorded changes in environmental conditions. The emphasis in the present volume, however, is largely on the role that the sediments play in controlling the chemistry of the oceans. The diagenetic changes that have the most immediate effect on the composition of seawater take place in the upper few metres of the sediment column. For this reason attention will be focused on these surface deposits and their role in biogeochemical cycles. The role played by sediments in recording palaeooceanographic change will be touched upon only briefly. It is, however, important to recognize that the oceans play a key role in the Earth System, a role that evolves over geological time, and the oceans also record the history of the evolution of the Earth System and its climate (e.g., Emerson and Hedges, 2008; Lenton and Watson, 2011).
In order to rationalize the process-orientated approach, special attention will be paid to a number of individual constituents, which can be used to elucidate certain key processes that play an important role in controlling the chemical composition of seawater. In selecting these process-orientated constituents it was necessary to recognize the flux-dominated nature of the seawater reservoir. The material fluxes that reach the oceans deliver both dissolved and particulate elements to seawater. It was pointed out above, however, that the amount of dissolved material in seawater is not simply the sum of the total amounts brought to the oceans over geological time. This was highlighted a long time ago by Forchhammer (1865) when he wrote:
Thus the quantity of the different elements in seawater is not proportional to the quantity of elements which river water pours into the sea, but is inversely proportional to the facility with which the elements are made insoluble by general chemical or organo-chemical actions in the sea…
[our italics]. According to Goldberg (1963), this statement can be viewed as elegantly posing the theme of marine chemistry, and it is this ‘facility with which the elements are made insoluble’, and so are removed from the dissolved phase, which is central to our understanding of many of the factors that control the composition of seawater. This was highlighted more recently by Turekian (1977). In an influential geochemical paper, this author formally posed a question that had attracted the attention of marine geochemists for generations, and may be regarded as another expression of Forchhammer’s statement, that is ‘Why are the oceans so depleted in trace metals?’ Turekian concluded that the answer lies in the role played by particles in the sequestration of reactive elements during every stage in the transport cycle from source to marine sink.
Ultimately, therefore, it is the transfer of dissolved constituents to the particulate phase, and the subsequent sinking of the particulate material, that is responsible for the removal of the dissolved constituents from seawater to the sediment sink. The biological production and consumption of particles by the ocean microbial community and its predators is central to this process. It must be stressed, however, that although dissolved → particulate transformations are the driving force behind the removal of most elements to the sediment sink, the transformations themselves involve a wide variety of biogeochemical processes. For example, Emerson and Hedges (2008) and Stumm and Morgan (1996) identified a number of chemical reactions and physicochemical processes that are important in setting the chemical composition of natural waters at a fundamental physicochemical level. These processes included acid–base reactions, oxidation–reduction reactions, complexation reactions between metals and ligands, adsorption processes at interfaces, the precipitation and dissolution of solid phases, gas–solution processes, and the distribution of solutes between aqueous and non-aqueous phases. The manner in which reactions and processes such as these, and those specifically associated with biota, interact to control the composition of seawater will be considered throughout the text. For the moment, however, they can be grouped simply under the general term particulate ↔ dissolved reactivity. The particulate material itself is delivered to the sediment surface mainly via the down-column sinking of large-sized organic aggregates as part of the oceanic global carbon flux. Thus, within the seawater reservoir, reactive elements undergo a continuous series of dissolved ↔ particulate transformations, which are coupled with the transport of biologically formed particle aggregates to the sea bed. Turekian (1977) aptly termed this overall process the great particle conspiracy. In the flux-dominated ocean system the manner in which this conspiracy operates to clean up seawater is intimately related to the oceanic throughput of externally transported, and internally generated, particulate matter. Further, it is apparent that several important aspects of the manner in which this throughput cycle operates to control the inorganic and organic compositions of both the seawater reservoir and the sediment sink can be assessed in terms of the oceanic fates of reactive trace elements and organic carbon.
Many of the most important thrusts in marine geochemistry over the past few years have used tracers to identify the processes that drive the system, and to establish the rates at which they operate (Broecker and Peng, 1982). These tracers will be discussed at appropriate places in the text. The tracer approach, however, also has been adopted in a much broader sense in the present volume in that special attention will be paid to the trace elements and organic carbon in the source/input → internal reactivity → sink/output transport cycle. Both stable and radionuclide trace elements (e.g. the use of Th isotopes as a ‘time clock’ for both transport and process indicators) are especially rewarding for the study of reactivity within the various stages of the cycle, and organic carbon is a vital constituent with respect to the oceanic biomass, the down-column transport of material to the sediment sink and sediment diagenesis.
To interpret the source/input → internal reactivity → sink/output transport cycle in a coherent and systematic manner, a three-stage approach will be adopted, which follows the cycle in terms of a global journey. In Part I, the movements of both dissolved and particulate components will be tracked along a variety of transport pathways from their original sources to the point at which they cross the interfaces at the land–sea, air–sea and rock–sea boundaries. In Part II, the processes that affect the components within the seawater reservoir will be described. In Part III, the components will be followed as they are transferred out of seawater into the main sediment sink, and the nature of the sediments themselves will be described. The treatment, however, is concerned mainly with the role played by the sediments as marine sinks for material that has flowed through the seawater reservoir. In this context, it is the processes that take place in the upper few metres of the sediments that have the most immediate effect on the composition of seawater. For this reason attention will be restricted mainly to the uppermost sediment sections, and no attempt will be made to evaluate the status of the whole sediment column in the history of the oceans.
The steps involved in the three-stage global journey are illustrated schematically in Fig. 1.1. This is not meant to be an all-embracing representation of reservoir interchange in the ocean system, but is simply intended to offer a general framework within which to describe the global journey. By directing the journey in this way, the intention therefore is to treat the seawater, sediment and rock phases as integral parts of a unified ocean system.