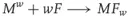![]()
Chapter 1
Introduction to Pyrolants
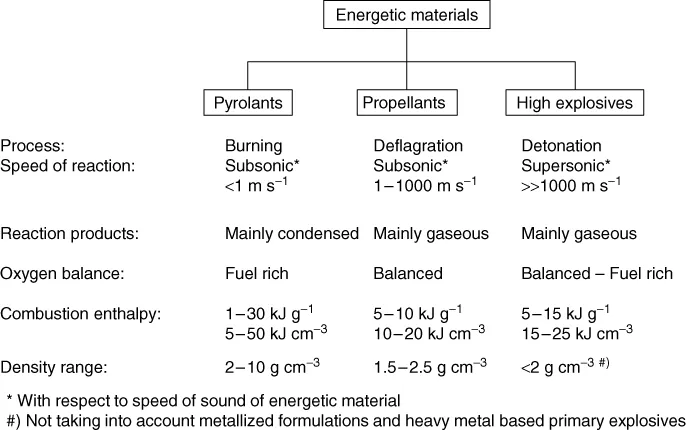
Energetic materials are characterised by their ability to undergo spontaneous (ΔG < 0) and highly exothermic reactions (ΔH < 0). In addition, the specific amount of energy released by an energetic material is always sufficient to facilitate excitation of electronic transitions, thus causing known luminous effects such as glow, spark and flame. Energetic materials are typically classified according to their effects. Thus, they can be classified into high explosives, propellants and pyrolants (Figure 1.1). Typical energetic materials and some of the salient properties are listed in Table 1.1.
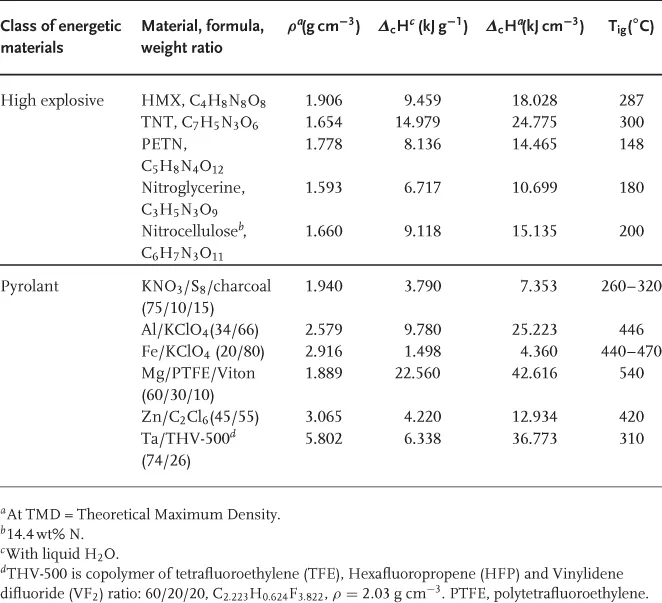
Table 1.1 Performance Parameters of Selected Energetic Materials.
When initiated, high explosives undergo a detonation. That is a supersonic shockwave supported by exothermic chemical reactions [1–3]. In contrast, propellants and pyrolants undergo subsonic reactions and mainly yield gaseous products as in the case of propellants [4, 5] or predominantly condensed reaction products as in the case of pyrolants. The term pyrolant was originally coined by Kuwahara to emphasise on the difference between these materials and propellants [6]. Thus, the term aims at defining those energetic materials that upon combustion yield both hot flames and large amount of condensed products. Hence, pyrolants often find use where radiative and conductive heat transfer is necessary. Pyrolants also prominently differ from other energetic materials in that they have both very high gravimetric and volumetric enthalpy of combustion and very often densities far beyond 2.0 g cm−3 (see Table 1.1 for examples).
Pyrolants are typically constituted from metallic or non-metallic fuels (e.g. Al, Mg, Ti, B, Si, C(gr) and S8) and inorganic (e.g. Fe2O3, NaNO3, KClO4 and BaCrO4) and/or organic (e.g. C2Cl6 and (C2F4)n) oxidizers or alloying partners (e.g. Ni and Pd). In contrast to propellants, they are mainly fuel rich and their combustion is influenced by afterburn reactions with atmospheric oxygen or other ambient species such as nitrogen or water vapour.
Pyrolants serve a surprisingly broad spectrum of applications such as payloads for mine-clearing torches (Al/Ba(NO3)2/PVC) [7, 8], delays (Ti/KClO4/BaCrO4) [9], heating charges (Fe/KClO4) [10, 11], igniters (B/KNO3) [12, 13], illuminants (Mg/NaNO3) [14, 15], thermites (Al/Fe2O3) [16, 17], obscurants (RP/Zr/KNO3) (RP, red phosphorus) [18], (Al/ZnO/C2Cl6) [20], tracers (MgH2/SrO2/PVC) [21], initiators (Ni/Al) [22] and many more. Recently, pyrolant combustion is increasingly used for the synthesis of new materials.
An important group of pyrolants are those constituted from metal powder and halocarbon compounds [19]. The high energy density of metal–halocarbon pyrolants stems from the high enthalpy of formation of the corresponding metal–halogen bond (M–X). Thus, chlorocarbon but mainly fluorocarbon compounds are used as oxidizers.
On the basis of metal fluorocarbon combinations, pyrolants show superior exothermicity compared to many of the aforementioned fluorine-free systems [22]. This advantage is due to the high enthalpy of formation of the metal–fluorine bond not outperformed by any other combination of the respective metal. Thus, the exothermic step
is the driving force behind the reaction (w = maximum valence).
Owing to a great number of metallic elemental fluorophiles (∼70), metal fluorocarbon pyrolants (MFPs) offer a great variability in performance. In addition, many alloys and binary compositions of fluorophiles may also come into play to further tailor the performance of the pyrolant: Mg4Al3, MgH2, MgB2, Mg3N2, Mg(N3)2, Mg2Si and so on [23]. Very often MFPs find use in volume-restricted applications where other materials would not satisfy the requirements – see, for example, payloads for infrared decoy flares (see Chapter 10). Within the scope of this book, the following applications are discussed:
- agent defeat payloads
- countermeasure flares
- cutting torches
- heating devices
- igniters
- incendiaries
- material synthesis
- obscurants
- propellants
- reactive fragments
- stored chemical energy propulsion systems
- tracers
- tracking flares
- underwater flares.
This book focuses only on specialised pyrotechnic applications; thus, for a more generalised introduction to pyrotechnics, the interested reader is referred to the books by Shidlovski [24], Ellern [25], McLain [26], Conkling [27, 28], Hardt [29] and Kosanke et al. [30].
References
1. Fickett, W. and Davis, W.C. (2000) Detonation – Theory and Experiment, Dover Publications Inc., Mineola, New York.
2. Zukas, J.A. and Walters, W.P. (1998) Explosive Effects and Applications, Springer Publishers, New York.
3. Cooper, P.W. (1996) Explosives Engineering, Wiley-VCH Verlag GmbH, New York.
4. Kubota, N. (2007) Propellants and Explosives, Thermochemical Aspects of Combustion, 2nd completely revised and extended edn, Wiley-VCH Verlag GmbH, Weinheim.
5. Assovskiy, I.G. (2005) Physics of Combustion and Interior Ballistics, Nauka, Moscow.
6. Kuwahara, T. and Ochiai, T. (1992) Burning rate of magnesium/TF pyrolants. Kogyo Kayaku, 53 (6), 301–306.
7. Kannberger, G. (2005) Test and Evaluation of Pyrotechnical Mine Neutralisation Means. ITEP Work Plan Project Nr. 6.2.4, Final Report, Bundeswehr Technical Center for Weapons and Ammunition (WTD 91), Germany.
8. N.N. (2005) Operational Evaluation Test of Mine Neutralization Systems, Institute for Defense Analyses, Alexandria, http://en.wikipedia.org/wiki/Political_divisions_of_the_United_States VA.
9. Wilson, M.A. and Hancox, R.J. (2001) Pyrotechnic delays and thermal sources. J. Pyrotech., 13, 9–30.
10. Callaway, J., Davies, N. and Stringer, M. (2001) Pyrotechnic heater compositions for use in thermal batteries. 28th International Pyrotechnics Seminar, Adelaide Australia, November 4–9, 2001, pp. 153–168.
11. Czajka, B. and Wachowski, L. (2005) Some thermochemical properties of high calorific mixture of Fe-KClO4. Cent. Eur. J. Energetic Mater., 2 (1), 55–68.
12. Klingenberg, G. (1984) Experimental study on the performance of pyrotechnic igniters. Propellants Explos. Pyrotech., 9 (3), 91–107.
13. Weiser, V., Roth, E., Eisenreich, N., Berger, B. and Haas, B. (2006) Burning behaviour of different B/KNO3 mixtures at pressures up to 4 MPa. 37th International Annual ICT Conference, Karlsruhe Germany, June 27–30, p. 125.
14. Beardell, A.J. and Anderson, D.A. (1972) Factors affecting the stoichiometry of the magnesium-sodium nitrate combustion reaction. 3rd International Pyrotechnics Seminar, Colorado Springs, CO, 21–25 August, pp. 445–459.
15. Singh, H., Somayajulu, M.R. and Rao, B. (1989) A study on combustion behaviour of magnesium – sodium nitrate binary mixtures. Combust. Flame, 76 (1), 57–61.
16. Fischer, S.H. and Grubelich, M.C. (1998) Theoretical energy release of thermites, intermetallics, and combustible metals. 24th International Pyrotechnics Seminar, Monterey CA, July 27–31, pp. 231–286.
17. Weiser, V., Roth, E., Raab, A., del Mar Juez-Lorenzo, M., Kelzenberg, S. and Eisenreich, N. (2010) Thermite type reactio...