
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Soil Chemistry
About this book
Provides comprehensive coverage of the chemical interactions among organic and inorganic solids, air, water, microorganisms, and the plant roots in soil
This book focuses on the species and reaction processes of chemicals in soils, with applications to environmental and agricultural issues. Topics range from discussion of fundamental chemical processes to review of properties and reactions of chemicals in the environment. This new edition contains more examples, more illustrations, more details of calculations, and reorganized material within the chapters, including nearly 100 new equations and 51 new figures. Each section also ends with an important concepts overview as well as new questions for readers to answer.
Starting with an introduction to the subject, Soil Chemistry, 5th Edition offers in-depth coverage of properties of elements and molecules; characteristics of chemicals in soils; soil water chemistry; redox reactions in soils; mineralogy and weathering processes in soils; and chemistry of soil clays. The book also provides chapters that examine production and chemistry of soil organic matter; surface properties of soil colloids; adsorption processes in soils; measuring and predicting sorption processes in soils; soil acidity; and salt-affected soils.
- Provides a basic description of important research and fundamental knowledge in the field of soil chemistry
- Contains more than 200 references provided in figure and table captions and at the end of the chapters
- Extensively revised with updated figures and tables
Soil Chemistry, 5th Edition is an excellent text for senior-level soil chemistry students.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
INTRODUCTION TO SOIL CHEMISTRY
No one regards what is at his feet; we all gaze at the stars. Quintus Ennius (239–169 BCE)Heaven is beneath our feet as well as above our heads. Henry David Thoreau (1817–1862)The earth was made so various that the mind of desultory man, studious of change and pleased with novelty, might be indulged. William Cowper (The Task, 1780)The Nation that destroys its soil destroys itself. Franklin Delano Roosevelt (1937)
1.1 The soil chemistry discipline

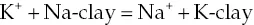
1.2 Historical background
Table of contents
- COVER
- TABLE OF CONTENTS
- PREFACE TO FIFTH EDITION
- PREFACE TO FOURTH EDITION
- ACKNOWLEDGMENTS
- 1 INTRODUCTION TO SOIL CHEMISTRY
- 2 PROPERTIES OF ELEMENTS AND MOLECULES
- 3 CHARACTERISTICS OF CHEMICALS IN SOILS
- 4 SOIL WATER CHEMISTRY
- 5 REDOX REACTIONS IN SOILS
- 6 MINERALOGY AND WEATHERING PROCESSES IN SOILS
- 7 CHEMISTRY OF SOIL CLAYS
- 8 PRODUCTION AND CHEMISTRY OF SOIL ORGANIC MATTER
- 9 SURFACE PROPERTIES OF SOIL COLLOIDS
- 10 ADSORPTION PROCESSES IN SOILS
- 11 MEASURING AND PREDICTING SORPTION PROCESSES IN SOILS
- 12 SOIL ACIDITY
- 13 SALT‐AFFECTED SOILS
- INDEX
- END USER LICENSE AGREEMENT
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app