Copper, Brass, and Bronze Surfaces
A Guide to Alloys, Finishes, Fabrication, and Maintenance in Architecture and Art
L. William Zahner
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Copper, Brass, and Bronze Surfaces
A Guide to Alloys, Finishes, Fabrication, and Maintenance in Architecture and Art
L. William Zahner
About This Book
A FULL-COLOR GUIDE FOR ARCHITECTS AND DESIGN PROFESSIONALS TO THE SELECTION AND APPLICATION OF COPPER, BRASS, AND BRONZE
Copper, Brass, and Bronze Surfaces, third in Zahner's Architectural Metals Series, provides a comprehensive and authoritative treatment of copper, brass, and bronze applications in architecture and art. If offers architecture and design professionals the information they need to ensure proper maintenance and fabrication techniques through detailed information and full-color images. It covers everything from the history of the metals and choosing the right alloy, to detailed information on a variety of surface and chemical finishes and corrosion resistance. The book also features case studies that offer strategies for designing and executing successful projects using copper, brass, and bronze.
Copper, Brass, and Bronze Surfaces is filled with illustrated case studies that present comprehensive coverage of how each metal is used in creating surfaces for building exteriors, interiors, and art finishes. All the books in Zahner's Architectural Metals Series offer in-depth coverage of today's most commonly used metals in architecture and art. This visual guide:
- Features full-color images of a variety of copper, brass, and bronze finishes, colors, textures, and forms
- Includes case studies with performance data that feature strategies on how to design and execute successful projects using copper, brass, and bronze
- Offers methods to address corrosion, before and after it occurs
- Explains the significance of the different alloys and the forms available to the designer
- Discusses what to expect when using copper, brass, and bronze in various exposures
Written for architecture professionals, metal fabricators and developers, architecture students, designers, and artists working with metals, Copper, Brass, and Bronze Surfaces offers a logical framework for the selection and application of copper, brass, and bronze in all aspects of architecture.
Frequently asked questions
Information
CHAPTER 1
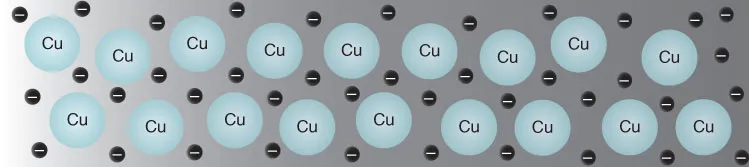
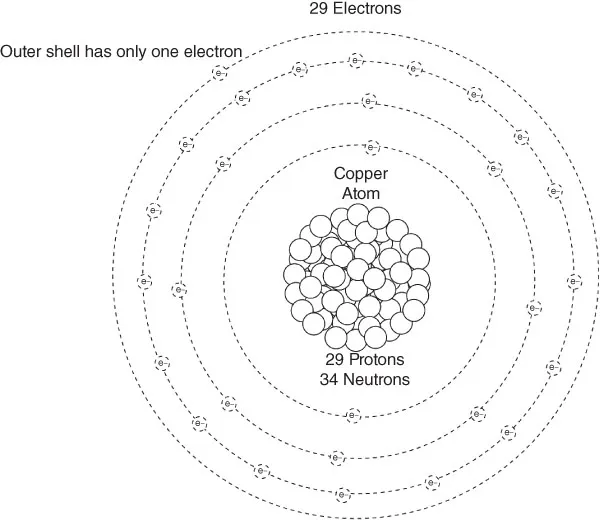
Introduction—Element 29
INTRODUCTION

| Metal | Siemens m−1 |
| Silver | 6.30 × 107 |
| Copper | 5.98 × 107 |
| Gold | 4.52 × 107 |
| Aluminum | 3.50 × 107 |
| Zinc | 1.68 × 107 |
| Nickel | 1.43 × 107 |
| Iron | 1.04 × 107 |
| Titanium | 1.80 × 106 |