
Environmental Toxicants
Human Exposures and Their Health Effects
Morton Lippmann, George D. Leikauf, Morton Lippmann, George D. Leikauf
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Environmental Toxicants
Human Exposures and Their Health Effects
Morton Lippmann, George D. Leikauf, Morton Lippmann, George D. Leikauf
About This Book
An Updated Reference on Human Exposure to Environmental Toxicants and A Study of Their Impact on Public Health
With the 4th edition of Environmental Toxicants: Human Exposures and Their Health Effects, readers have access to up-to-date information on the study and science of environmental toxicology and public health worldwide. Practitioners and professionals can use this resource to understand newly discovered information on the adverse health effects of toxins and pollutants in air, water, and occupational and environmental environments on large human populations.
The 4th edition of this book is updated to reflect new knowledge and research on:
? Performing risk assessments on exposed individuals
? Assessing the effects of toxicants and substances on large populations for health and medical professionals
? Patterns of human exposure to select chemical toxicants
? World Trade Center dust, agents for chemical terrorism, and nanoparticles
For health professionals, including health authorities, public health officials, physicians, and industrial managers, who are seeking new research and techniques for managing environmental substances, this invaluable reference will guide you through in a thorough, easy- to-read manner.
Frequently asked questions
Information
1
INTRODUCTION AND BACKGROUND
1.1 CHARACTERIZATION OF CHEMICAL CONTAMINANTS
1.1.1 Concentration Units
1.1.2 Air Contaminants
1.1.2.1 Gases and Vapors
1.1.2.2 Aerosols
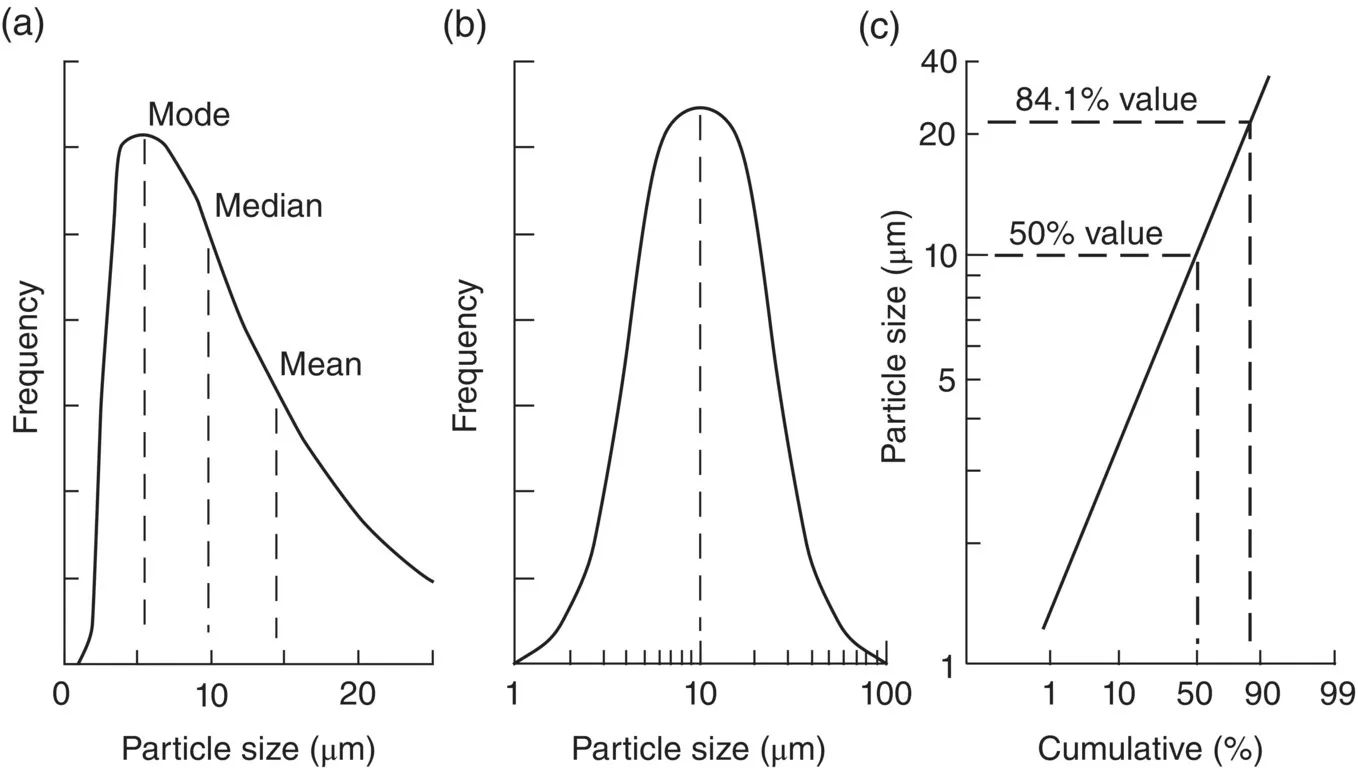
1.1.3 Particle Characteristics
- Surface: For spherical particles, the surface area (A) varies as the square of the radius (r...