
eBook - ePub
Rates and Equilibria of Organic Reactions
As Treated by Statistical, Thermodynamic and Extrathermodynamic Methods
- 474 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Rates and Equilibria of Organic Reactions
As Treated by Statistical, Thermodynamic and Extrathermodynamic Methods
About this book
Graduate-level text stresses extrathermodynamic approach to quantitative prediction and constructs a logical framework that encompasses and classifies all known extrathermodynamic relationships. Numerous figures and tables. Author and Subject Indexes.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Rates and Equilibria of Organic Reactions by John E. Leffler,Ernest Grunwald in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Chemistry. We have over one million books available in our catalogue for you to explore.
Information
7
Extrathermodynamic Free Energy Relationships. I. Substituent Effects
He was pinched perspiringly in the epistemological dilemma of the skeptic, unable to accept solutions to problems he was unable to dismiss as unsolvable. He was never without misery and never without hope.
Joseph Heller
Catch 22
Catch 22
In Chapter 6 we constructed a formal system for generating extrathermodynamic relationships, based on the concept of physical interaction mechanisms with scalar independent variables. We shall now apply this system to the analysis of substituent effects. We shall find that simple linear free energy relationships need not be limited to closely related reactions but in some cases encompass entire classes of reactions. Thus our formal interaction mechanisms have exact counterparts in real interactions of broad scope.
When a linear free energy relationship applies to a group of reactions, we may choose the model process to be any convenient reaction in the group under a suitable single set of conditions. Normally we would choose the model process on the basis that accurate data are already available for a large number of substituents. Yet even so, we usually find that the existing data do not cover a sufficiently long list of structural changes. We therefore supplement the data for the model process with structural parameters whose operational definition varies somewhat from one structural change to another. For example, the temperature, the solvent, or even the nature of the reaction may be different, although an attempt is made to hold these variations to a minimum. In addition, an attempt is made to show that the parameters obtained from these variants of the standard or reference process are not very different from those that would have been obtained under fixed conditions.
THE HAMMETT ρσ RELATIONSHIP
The best known of the extrathermodynamic equations relating reaction rate or equilibrium to the structure of the reagent is the Hammett ρσ equation.148 It describes the effect of a meta- or para-substituent on the rate or equilibrium constant of an aromatic side-chain reaction. It is based on the fact that, as the substituent is varied, the logarithms of the rate or equilibrium constants for a large number of aromatic side-chain reactions are linearly related to one another. Although any one of these reactions could have been used to define a set of substituent parameters in terms of which the free energy changes of the others might be described, the parameter σ has been defined on the basis of the acid dissociation equilibrium of benzoic acid in water at 25°.

(1)
The reason for this choice was the accessibility of the data and the belief, current at that time, that the mechanisms by which substituents exert their effects were known for this reaction. The substituents, X, must be in the meta- or para-position, since the linear relationships fail for ortho-substituents.
The effect of substitution on the rate or equilibrium constants for other benzene side-chain reactions can now be expressed as a function of σ. The resulting equation (2) is the well-known Hammett equation.
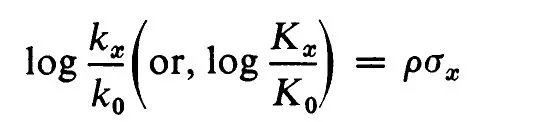
(2)
The constants kx and k0 (or Kx and K0) must be for the same experimental conditions, though not necessarily the conditions used in the definition of σ. The constant ρ is a function of the reaction and will also vary with the reaction conditions.
Values of the parameter σ for use in the Hammett equation are listed in Table 7-1.149,150 Whenever possible, the values have been based on data for the standard model process, as defined in equation 1. Values obtained in this way will be referred to as primary σ-values. When the required data for the standard process are not available, the σ-value for that substituent is obtained from some secondary reference process, chosen to resemble the ionization of benzoic acid in water at 25° as closely as possible. The procedure for obtaining such a secondary σ-value is as follows. First, a number of known primary σ-values are used to evaluate...
Table of contents
- Title Page
- Copyright Page
- Apologia
- Preface
- Table of Contents
- Glossary of Symbols
- I - Equilibrium from the Statistical Point of View
- 2 - Equilibrium and the Gibbs Free Energy
- 3 - Free Energy, Enthalpy, and Entropy
- 4 - Concerning Rates of Reaction
- 5 - Rates of Interconversion of Subspecies
- 6 - Theoretical Introduction to Extrathermodynamic Relationships
- 7 - Extrathermodynamic Free Energy Relationships. I. Substituent Effects
- 8 - Extrathermodynamic Free Energy Relationships. II. Medium Effects
- 9 - Extrathermodynamic Analysis of Enthalpy and Entropy Changes
- 10 - Some Mechanochemical Phenomena
- Authors Index
- Subject Index
- A CATALOG OF SELECTED DOVER BOOKS IN ALL FIELDS OF INTEREST
- A CATALOG OF SELECTED DOVER BOOKS IN ALL FIELDS OF INTEREST