
eBook - ePub
Glucuronidation of Drugs and Other Compounds
Geoffrey Dutton
This is a test
Share book
- 268 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Glucuronidation of Drugs and Other Compounds
Geoffrey Dutton
Book details
Book preview
Table of contents
Citations
About This Book
Published in 1980: In a previous publication on glucuronic acid both free and conjugated, the author expressed the hope that glucuronic acid studies over the following few years might expand vigorously. The have expanded, and none more vigorously that the study of biosynthesis of simple glucuronides.
Frequently asked questions
How do I cancel my subscription?
Can/how do I download books?
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
What is the difference between the pricing plans?
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
What is Perlego?
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Do you support text-to-speech?
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Is Glucuronidation of Drugs and Other Compounds an online PDF/ePUB?
Yes, you can access Glucuronidation of Drugs and Other Compounds by Geoffrey Dutton in PDF and/or ePUB format, as well as other popular books in Medicine & Pharmacology. We have over one million books available in our catalogue for you to explore.
Information
Part I
Glucuronidation, Glucuronides, and Studies on UDPGlucuronyltransferase In Vitro
Chapter 1
Introduction — The Biological Function of Glucuronidation
I. What Glucuronidation Is
Glucuronidation is the most widespread form of “conjugation” in mammalian metabolism. “Conjugation” is a synthetic reaction involving the coupling in vitro of two molecules, usually with elimination of water. There are ten major conjugation reactions in mammals,2 and many others exist, in both animals and plants.3 In glucuronidation, the sugar acid, D-glucuronic acid (Figure 1), is coupled with a wide variety of compounds (see Chapter 2, Sections I and II) to form glycosides, the β-D-glucopyra-nosiduronic acids or, as termed throughout this book except where confusion could arise, the glucuronides.4
II. “Detoxication” Reactions
Conjugation is best known as a “detoxication” reaction. The validity of the term “detoxication” is discussed below, but the process can be considered as a progressive increase in polarity of a molecule. This increased polarity leads to its solubility in bile or urine and its consequent excretion from the body. The authority on detoxication, R. T. Williams, conveniently divided detoxication into two phases.5 Phase 1 consists of reactions such as oxidations, reductions, and hydrolyses whereby the molecule achieves a “handle”, a polar group such as -OH, -NH2, or -COOH. It is then able to enter Phase 2, where the “handle” takes part in conjugation. In Phase 2, conjugation with a highly polar molecule or ion such as glucuronic, sulfuric, or acetic acids usually ensures rapid excretion.5
A classical example, the detoxication of the drug phenacetin, is shown in Figure 2. It is quoted by Williams5 from the work of Smith and Williams.7
In Phase 1, the neutral lipid-soluble phenacetin is oxidatively deethylated to 4-acet-amidophenol. This compound is sparingly soluble in water, with a pKa of about 10, and is 0.25% dissociated at the body pH of 7.4.7 In Phase 2, the 4-acetamidophenol is conjugated with glucuronic acid to give the glucuronide, a strong acid of pKa 3.5, highly water soluble, and 99.99% dissociated at pH 7.4. 4-Acetamidophenyl glucuronide is readily excreted by the kidney. The body is, therefore, cleared of the drug by metabolism through Phases 1 and 2. A given compound (e.g., a phenol) may be ingested in a form already suitable for metabolism by Phase 2 enzymes. In that case, detoxication need not involve any Phase 1.
III. Distinctive Aspects of the Phase 2 Reactions of Detoxication
Phase 2 reactions are distinct from Phase 1 reactions. First, different sets of enzymes are involved in the two phases. Second, the products of Phase 1 reactions are still partially lipid soluble; many possess biological activity, either toxic or therapeutic. Thus 4-acetamidophenol, the Phase 1 metabolite of phenacetin noted above, is an active drug, the activity of administered phenacetin being largely due to this metabolite.7 Products of Phase 2 are usually, but not always, much less active, or quite inactive, biologically. This is due to two factors: (1) increased water solubility and decreased lipid solubility, allowing rapid removal from the body; (2) masking of biologically-active groups by superimposition of, or stereochemical hindrance by, the conjugating molecule. 4-Acetamidophenyl glucuronide in Figure 1 exhibits none of the antipyretic or analgesic properties of the administered phenacetin or its Phase 1 product.
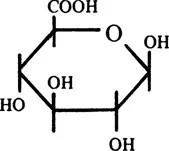
FIGURE 1. Structure of D-glucuronic acid.
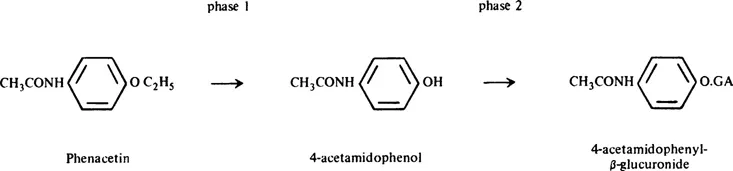
FIGURE 2. The detoxication of phenacetin.
Third, Phase 2 reactions are all synthetic reactions involving expenditure of energy. This energy can be applied by two methods to ensure conjugation. The first, and principal, method forms a “high-energy” endogenous molecule which donates the conjugating group, e.g., in formation of acetyl-coenzyme A, which later donates an acetyl group to the molecule, conjugating it as an acetate. Another example is the formation of UDPglucuronic acid, the donor of glucuronic acid.
The second method involves activation of the xenobiotic molecule to be conjugated. This is most common in conjugations with amino acids, as that of administered benzoic acid with glycine to form benzoylglycine (hippuric acid).
IV. The Concept of Detoxication
We must examine more closely the term “detoxication”. The use of the word “detoxication” presumably implies that toxicity of a molecule or ion to a particular species has been lessened or abolished. It would apply equally to molecules of endogenous and of exogenous origin, to conversion of ammonia to urea as much as to conversion of phenacetin to 4-acetamidophenyl glucuronide. Generally, the term is reserved for originally exogenous, usually lipid soluble, molecules. Such compounds, if not utiliz-able as fuel or building material by the species in question (i.e., if they are anutrients), will be metabolized by Phase 1, Phase 2, or both. The resulting metabolites will usually be more water soluble and less lipid soluble. They will, therefore, when once in blood or bile, remain there and not readily pass through lipid membranes into cells. From blood, they will be filtered by the kidney and pass down the tubules without reabsorption by the tubule cells, to appear almost quantitatively in the urine. The increased water-solubility and pKa values of some administered compounds and their glucuron-ides have been listed.6,8
We can now reconsider the concept of detoxication. Any detoxication of compounds X and Y that we see is merely an accompaniment of the metabolism of X and Y. X and Y enter the body and are oxidized, reduced, hydrolyzed, conjugated, or otherwise treated according to their chemical structure. Their structure alone determines what Phase they enter and by which of the host’s enzymes they are accepted. Their structure determines the structure of their metabolite, and so its solubility in water, its ease of excretion, and its toxicity. There is no guarantee that these metabolites will be less toxic than X or Y, and hence, no guarantee that X and Y will be truly detoxicated by the biphasic metabolism loosely termed “detoxication”.
This might be thought obvious. Yet much confusion, and several unfortunate incidents, have resulted from the assumption that th...