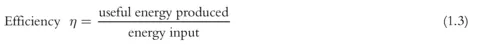![]()
1 Energy and climate protection
The term 'energy'
We hear the word ‘energy’ quite often without thinking about it much. It is used in a lot of different contexts. For instance, the term can be used in the sense of vitality, and we say that people have ‘a lot of energy’ to describe their temperament.
This book only deals with forms of – especially renewable – energy produced by technology, and they are described in terms of the laws of physics. Energy and power are two nearly inseparable terms. But because they are often confused, a clarification of the distinction between the two and other related terms is a good starting point.
In general, energy describes a system’s ability to produce external effects, such as force along a path. A body’s energy can change by taking up or exerting work. Here, energy occurs in a wide range of forms, including:
- mechanical energy
- potential energy
- kinetic energy
- heat or thermal energy
- magnetic energy
- mass
- electrical energy
- radiant energy
- and chemical energy.
In the definitions above, a litre of gasoline is a type of stored energy; when it is combusted, the force of an engine can move a car (having a certain mass) for a certain distance. The motion of the car is then a kind of work.
Heat is also a type of energy. We see its effects when the hot air rising from a candle moves the parts of a hanging mobile. A force is also needed for this motion.
The wind also contains energy, which can be used, say, to turn the blades of a wind turbine. The sun’s rays can be used to generate heat. Radiation, especially solar radiation, is another form of energy.
Power is defined as the amount of work W done over time t.
It therefore indicates how much time it took to perform a certain amount of work. For instance, work can be a person lifting a sack of cement up one metre. The work performed increases the sack’s potential energy. If the sack is lifted twice as quickly, less time is needed, but the amount of power doubles although the same amount of work is performed.
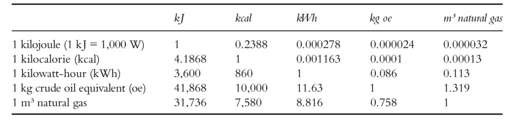
Energy and work are expressed in units derived from the SI units of J (joules), Ws (watt-seconds), and Nm (newton metres). Power is measured in W (watts). Table 1.1 shows the conversion factors for the main units used today for energy equipment. In addition, there are a number of antiquated energy units, such as kilopond metres, kpm (1 kpm = 2.72 · 10–6 kWh); erg (1 erg = 2.78 · 10–14 kWh); electron volts (still used in physics), eV (1 eV = 4.45·10–26 kWh); and (still common in the US) Btus (British Thermal Unit, 1 Btu = 1055.06 J = 0.000293071 kWh).
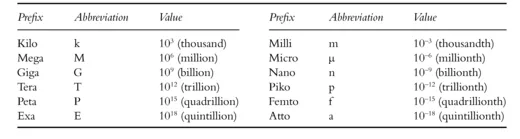
Because values can often be very small or very large and exponential indications are hard to follow, a number of prefixes are used as shown in Table 1.2.
Often, terms describing energy and power are confused along with the units defining them. When the wrong sizes are used, meanings change, leading to misunderstandings in the best case.
Table 1.1 Conversion factors for various energy units
Table 1.2 Prefixes and prefix abbreviations
For example, let’s take a newspaper article from the 1990s about a house with a solar roof. The article describes a photovoltaic system with a total output of 2.2 kW. The author then says that the price paid per kW of electricity sold to the grid was very low at 0.087 Deutschemarks, equivalent to roughly 0.05 cents today. But if the system were paid based on its power (kW represents the installed capacity), the entire system would receive 2.2 kW × €0.087/kW = €0.19. The compensation for solar power was inadequate for a long time, to be sure, but probably no system owner had to make do with less than €0.20. Here, the author was actually speaking of the electric energy sold to the grid and compensated at a rate of €0.087 per kilowatt-hour (kWh). If the system produced 1,650 kWh to sell to the grid, the owner would be paid €143.55 – 750 times more. This anecdote shows what a big difference a little ‘h’ can have if it is missing.
According to the laws of physics, energy cannot be generated, destroyed, or lost. Nonetheless, we speak of energy losses, energy generation, and energy production – despite the law of conservation of energy, which states:
Within an isolated system, total energy remains constant. Energy cannot be destroyed or created out of nothing; it can, however, be converted into various forms and exchanged between various parts of the system.
To see how energy can be converted from one form into another, let’s go back to the example of gasoline and cars. Gasoline is a type of stored chemical energy. When it is combusted, thermal energy is created. The motor turns this thermal energy into kinetic energy, which is passed on to the car. When all of the gasoline has been consumed, the car stops. The energy has not, however, disappeared; rather, it was converted into potential energy (to the extent there is a difference in altitude), into the motor’s waste heat, into friction (the tires), and given off as heat to the surroundings. We cannot, however, make use of this ambient heat in general. While driving, we converted most of the useful energy contained in the gasoline into ambient heat, which cannot be used. From our perspective, this energy is lost. When we speak of ‘lost’ or ‘consumed’ energy, we mean that we have converted high-quality energy into energy of lower, generally not usable quality.
The situation is different with photovoltaics. It converts sunlight directly into electrical energy. In common parlance, we say that the solar energy system ‘generates electricity’. This description is incorrect within the laws of physics. It would be correct to say that the photovoltaic system converts a form of energy we cannot easily use (solar radiation) into higher-quality energy (electricity).
In this conversion, the energy can be used at different levels of efficiency. To illustrate how this works, let’s take the example of boiling water.
The thermal energy Q needed to heat up a litre of water ( m = 1 kg) from temperature ϑ1 = 15 °C to ϑ2 = 98 °C is relative to the heat capacity c of water cH2O = 4.187 kJ/(kg K), giving us
to produce Q = 348 kJ = 97 Wh.
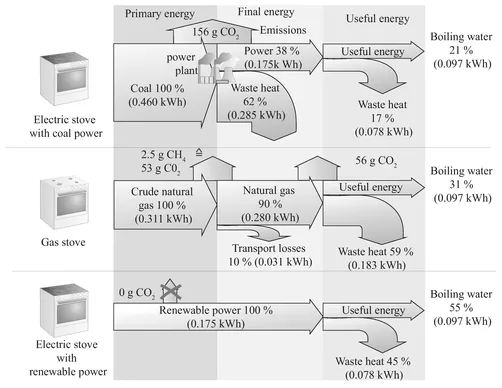
A consumer magazine compared different water-heating appliances. The results are shown in Figure 1.1. In addition to various electrical appliances, gas stoves were also included. The comparison shows that gas stoves are the worst in terms of energy consumption even though its energy costs are the lowest. This difference comes about because we are comparing different types of energy here.
The electric stove uses electric energy to heat up the water. Electricity is quite rare in nature; natural occurrences include lightning and eels that use electrical charges to stun attackers. Electricity therefore first has to be made from another source of energy, such as coal in a power plant. Once again, an enormous amount of waste heat is created in the process and passed on to the ambient environment. In other words, only a small part of the energy in the coal is converted into electrical energy, with the rest lost as waste heat. The quality of the conversion is referred to as efficiency η and is defined as follows:
Figure 1.1 ‘What it costs to boil water’ from 1994 [Sti94]
The average efficiency of a thermal power plant in Germany in the 1990s was around 34 % [Hof95]. The efficiency of modern power plants is slightly higher. Nonetheless, roughly 60 % of the energy consumed is nonetheless lost as waste heat when only 40 % comes out of the plant as electrical energy.
When energy technologies are used, a distinction therefore needs to be made between various stages of energy conversion: primary energy, final energy, and useful energy, as shown in Table 1.3.
The previously calculated amount of heat thus represents useful energy, while the numbers in Figure 1.1 are final energy. But when we compare the efficiency of gas and
Table 1.3 Primary energy, final energy, and useful energy
| Term | Definition | Energy type or source |
|
| Primary energy | Energy in its original form, not technically processed | Such as crude oil, coal, uranium, solar radiation, and wind |
| Final energy | Energy as provided to consumers | Such as natural gas, heating oil, fuel, electricity, and district heat |
| Useful energy | Energy as it is consumed | Such as artificial lighting, heat, drive energy for machines and vehicles |
Figure 1.2 Energy conversion chain, losses and carbon dioxide emissions from boiling water
electricity, we should start with primary energy because both of them are quite different forms of final energy.
For electricity, we need to start with the source of energy fed into the plant, such as coal. Natural gas used to heat water is also final energy. When natural gas is transported to consumers, there are also losses, though they are minor in comparison to a power plant generating electricity. We then find that the electric stove’s primary energy consumption is 460 Wh = 1,656 kJ, some 50 % greater than the gas stove’s consumption although final energy consumption is more than 30 % lower. Figure 1.2 shows another comparison of the energy conversion chains based on the example of water heated by an electric stove and a gas stove.
Clearly, gas stoves are better for water heating than electrical stoves if the electricity is made with conventional fuels in terms of primary energy consumption, the decisive factor for environmental impacts. This example illustrates how important the distinction between primary energy, final energy, and useful energy is. Otherwise, we draw incorrect conclusions, as the comparison of gas and electric stoves in Figure 1.1 shows.
Trends in energy demand
Trends in global energy demand
At the end of the eighteenth century, crude oil and coal were still marginal sources of energy. Wood still covered most of the energy demand for heat. Progress had, however, been made in the use of water and wind. Both of them were used in mills and pump water.
In 1769, James Watt invented a useful steam engine, thereby opening the door to industrialization. Gradually, steam engines – and later, combustion engines – replaced wind and water mills. Coal became the biggest source of energy, followed by crude oil starting at the beginning of the twentieth century as automobiles became more common. As a source of energy, firewood increasingly became marginal in industrialized countries. And whereas traditional water mills fit well into landscapes, modern hydropower became giant technical facilities.
After the Great Depression of 1929, energy consumption rose ...



![Figure 1.1 ‘What it costs to boil water’ from 1994 [Sti94]](https://book-extracts.perlego.com/1558291/images/fig00007-plgo-compressed.webp)