
eBook - ePub
Solution Mining
Leaching and Fluid Recovery of Materials
- 470 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
First published in 1998. This book offers a wealth of information on the rapidly expanding field of solution mining: yhe extraction of materials from the earth by leaching and fluid recovery. This is an introductory text for students and professional engineers that is comprehensive and emphases current practice and theory. Percolation leaching of fragmented ground is covered, as well as true and modified in situ teaching. Solution mining of gold, copper and uranium ores, several slats extracted from evaporates and brines, and sulfur are discussed. Mineral teaching chemistry and kinetics, hydrology (including flow equations for various wellfields and other fluid recovery systems), environmental containment and solution mining simulation models are also included.
Trusted by 375,005 students
Access to over 1 million titles for a fair monthly price.
Study more efficiently using our study tools.
Information

INTRODUCTION
Nearly 30% of domestic copper mine production is derived by solution mining methods. An important copper contributor is percolation leaching of huge waste rock dumps, resulting from the stripping of overburden at existing open pit copper mines. Most of the copper in mine waste is in the form of the primary copper sulfide mineral, chalcopyrite. Because of its greater complexity, copper mine waste leaching will be discussed in the next chapter. Percolation leaching of oxidized copper ores is also important and will be covered in this chapter. Leaching secondary copper sulfide minerals will also be covered in this chapter.
Natural occurring copper ores usually do not have adequate permeability for percolation leaching without fragmentation (rubblizing). Copper in sandstone is an exception but of minor economic importance. Percolation leaching of copper can occur in heaps, mine waste dumps, and caved mine workings above the water table. Large explosive blasts of ore have been conducted to adequately rubblize copper ore prior to percolation leaching.
An important difference between copper percolation leaching and gold percolation leaching is the much greater concentrations of copper metal in both the ore and the resulting pregnant solution. Typical ore and solution grades are compared in Table 5.1. The copper grades in both cases are
Table 5.1 Typical Ore and Solution Grades
| Percolation Leaching | Solids Grade (ppm) | Solution Grade (ppm) |
| Gold | 1–3 | 0.2 |
| Silver | 20–75 | 5–40 |
| Copper | 2000–4000 | 1000 |
| (0.2–0.4%) | (2 lb/ton) |
between 1,000 and 10,000 times greater than the gold grades. This increases the time required to complete leaching extractions of copper for similar rock sizes and microporosities, because more material must be transported out of the rock through its solution-filled micropores.
COPPER ORE DEPOSIT GEOLOGY
Copper ore deposits are sedimentary, stratiform or porphyritic. In porphyry deposits, copper is disseminated either in fine grained igneous intrusions or adjacent host rock. This is the dominant type of copper ore deposit in the western hemisphere. Figure 5.1 illustrates an idealized vertical section through a porphyry copper ore deposit, showing primary sulfide ore at
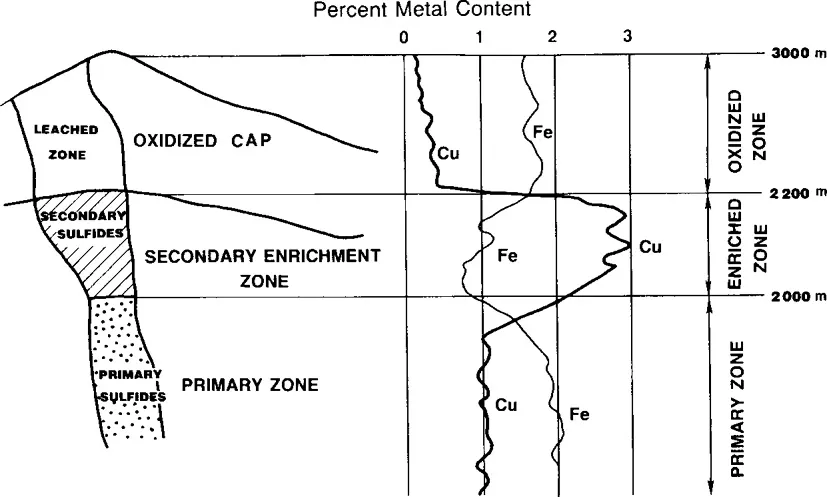
Figure 5.1. Copper porphyry ore deposit types showing primary, secondary enriched and oxide zones (Wadsworth, 1987).
depth, an intermediate secondary enrichment zone and, at the top, a naturally weathered (leached) zone above the water table where all of the copper has been oxidized, given sufficient time. Typical copper concentrations (weight percent) in the ore in each zone are also shown in Fig. 5.1. Because of subsequent erosion, all of these zones may not be present and sometimes there are mixed oxide/sulfide zones in a copper ore deposit.
The primary sulfide mineral is chalcopyrite (CuFeS2) and there may be several secondary sulfide minerals. Pyrite (FeS2) is always present in primary mineral zones and it is usually present in secondary mineral zones. Oxidized iron minerals are usually present in the oxidized zone (see Fig. 5.2). Water flooding excludes air (oxygen) and, consequently, the oxidized ore zone cannot extend below the water table, at least as it existed historically over the life of the mineral deposit.
Oxidized mineral zones are often depleted by the leaching action of percolating supergene water. If pyrite was previously abundant, acidic solutions were generated that solubilized copper and transported it downward. Precipitation of the soluble copper below the water table causes
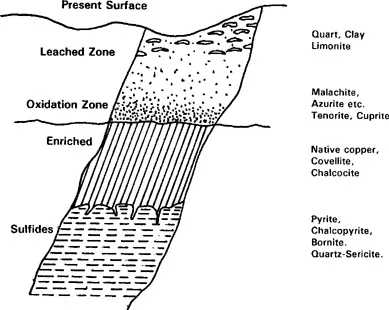
Figure 5.2. Upper portion of copper ore deposit showing zonation, water table, and typical copper minerals (Dudas, 1974).
secondary copper enrichment, as the oxidation potential drops and chalco-pyrite reacts with the infiltrating soluble copper to form secondary minerals, e.g., chalcocite (Cu2S), bornite (Cu5FeS4), and covellite (CuS).
If acidic conditions cannot be maintained in the oxidized zone, copper carbonate minerals may precipitate. Hence, there are several possible oxidized copper minerals, many of which are shown in Fig. 5.2. Ores containing these minerals as the predominant copper source are referred to as “oxide copper ores.”
Figure 5.3 illustrates the Eh-pH relations for the copper-iron-sulfur-water system (Wadsworth, 1987). The secondary enriched zone corresponds to the Eh region of approximately 0.2–0.6 V, at low pH, with the oxidized zone above this Eh range and the primary mineral zone below it. Autotrophic bacteria, such as Thiobacillus ferrooxidans, catalyze both sulfide mineral oxidation and oxidation of dissolved ferrous ions to ferric ions. This is very important to the percolation leaching of copper sulfide minerals, and it will be discussed in the next chapter. From Fig. 5.3, note that the bacteria are
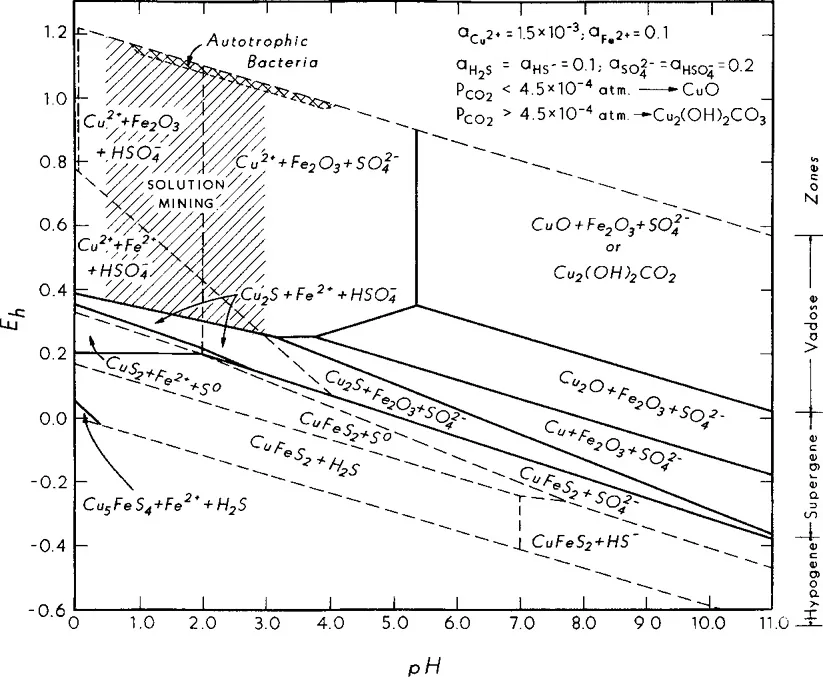
Figure 5.3. Eh-pH diagram for the copper-iron-sulfur-water system showing zoning, solution mining region and region of bacteria action (Wadsworth, 1987).
only active at high oxidation potentials, corresponding to a minimum of 2–3% oxygen in air, and about 1 ppm of dissolved oxygen in the leachate.
CONSEQUENCES OF DIFFERENT COPPER ORE TYPES
As a practical matter, it is useful to distinguish oxide copper ores, both with and without secondary sulfides discussed in this chapter, from primary ores and primary mineralization mine waste, which are considered in the next chapter. Oxide copper minerals leach rapidly in acid, when compared with the other copper minerals, while secondary sulfide minerals require oxidation and leach at a slower rate. Nevertheless, the secondary copper sulfide minerals oxidize and dissolve much more rapidly than either chalcopyrite or pyrite, and they are often called “leachable sulfides.” Also, much less oxidizing reagent (dissolved oxygen or ferric ions) is required to leach them than to leach primary copper ore. Chalcopyrite in primary ore (or mine waste) leaches very slowly, and at about the same rate as pyrite, which is usually the most prevalent sulfide mineral in primary ore. Consequently, when leaching these primary ores, most of the oxidizing reagent is consumed to oxidize pyrite rather than chalcopyrite and very large amounts of oxygen are required relative to the amount of copper extracted.
The different ore types cause significant differences between the heap leaching practice for oxide copper minerals and for primary copper ore (chalcopyrite). Some of these differences in the criteria for percolation leaching practice, which are discussed in greater detail in this and the subsequent chapter, a...
Table of contents
- Cover
- Half Title
- Full Title
- Copyright
- Dedication
- Contents
- List of Symbols
- Preface
- ONE Introduction
- TWO Heap Leaching Gold (Silver) Ore—Theory
- THREE Heap Leaching Practice (With Applications to Gold and Silver Ores)
- FOUR Environmental Control in Percolation Leaching
- FIVE Percolation Leaching Oxidized and Secondary Sulfide Copper Minerals
- SIX Percolation Leaching Copper Mine Waste (Primary Sulfides)
- SEVEN Solution Flow During Percolation Leaching of Ore Heaps
- EIGHT Oxygen Diffusion and Air Flow During Heap Leaching
- NINE Biooxidation Heap Pretreatment of Sulfide Refractory Gold Ore and High-Sulfur Coal
- TEN In Situ Flooded Leaching
- ELEVEN In Situ Leaching Hydrology
- TWELVE Numerical Simulation of Fragmented Rock Leaching
- THIRTEEN Evaporites, Brine and Sulfur
- FOURTEEN Solar Evaporation Ponds
- FIFTEEN Remediation of Contaminated Ground and Water
- Appendix
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Solution Mining by Robert Bartlett,Robert W. Bartlett in PDF and/or ePUB format, as well as other popular books in Business & Business General. We have over one million books available in our catalogue for you to explore.