
- 536 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Fluid-Solid Reactions
About this book
Fluid-Solid Reactions, Second Edition takes a detailed and thorough look at the scope of fluid-solid reaction systems, focusing on the four phenomena: external mass transfer, pore diffusion, chemical reaction, and adsorption/desorption. This completely revised new edition builds on the classic original edition through the introduction of cutting-edge new theories and applications, including the formulation and application of a new and convenient law that governs fluid-solid reaction kinetics. This book will be of primary interest to practicing engineers engaged in process research, development, and design in the many fields where fluid-solid reactions are critical to workflow and research.
Fluid-solid reactions play a major role in the technology of most industrialized nations. These reactions encompass a very broad field, including the extraction of metals from their ores, the combustion of solid fuels, coal gasification, and the incineration of solid refuse.
- Features 50% new and revised content, arming researchers with the latest developments in the field
- Details a new unified approach to modeling the rates of fluid-solid reaction systems
- Authored by one of the world's foremost experts on fluid-solid reactions and their applications in the field
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Introduction
Abstract
Keywords

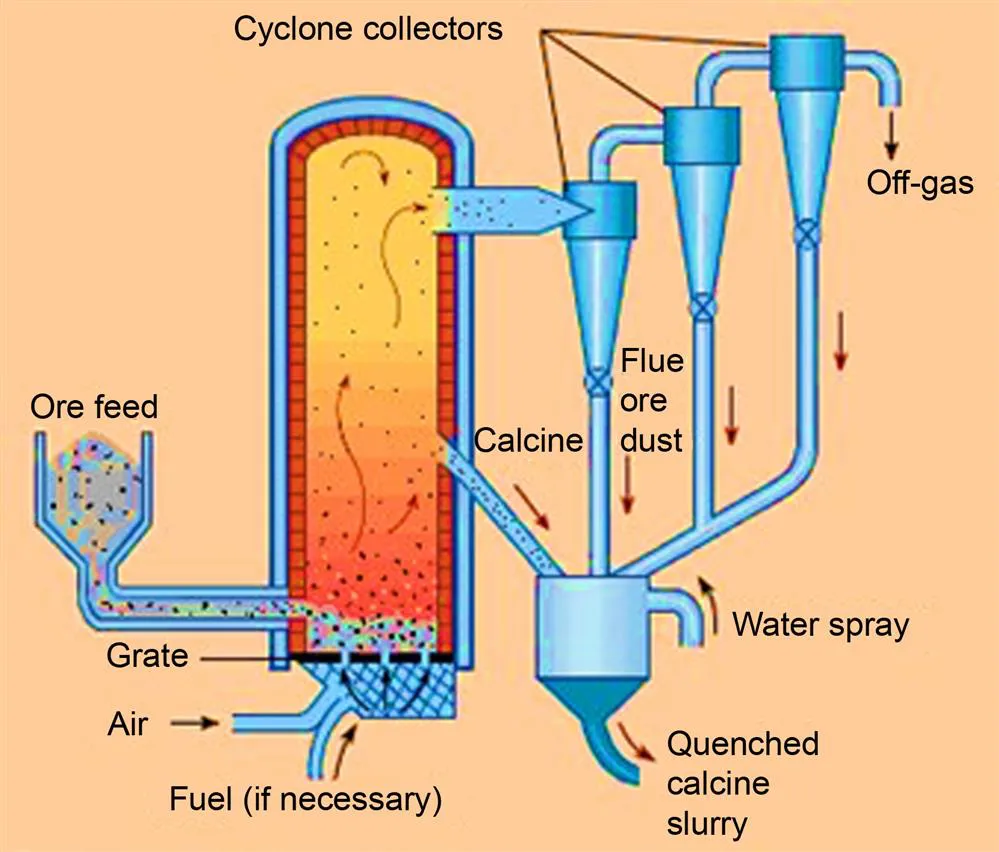

- A. Solid→Fluid
- B. Solid 1→Fluid+Solid 2
- C. Fluid+Solid→Fluid
- D. Fluid+Solid 1→Fluid+Solid 2
- E. Fluid+Solid 1→Solid 2
- F. Solid 1+Solid 2→Fluid+Solid 3
- G. Solid 1+Solid 2→Solid 3+Solid 4 (if proceeding through fluid intermediates)
- H. Fluid→Fluid+Solid
- I. Fluid→Solid
- 1.
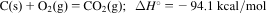
- 2.
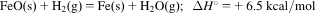
- 3.
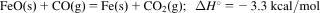
- 4.
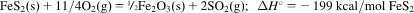
- 5.
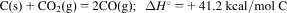
- 6.
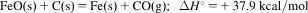
- 7. 4CuFeS2 (s)+17O2 (dissolved in liq.)+4H+ (in liq.)=4Cu2+ ()+4Fe3+ (
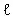 )+
)+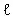 (
(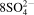 )+2H2O (
)+2H2O (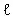 )
)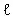
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Dedication
- Preface
- Acknowledgments
- Chapter 1. Introduction
- Chapter 2. Constitutive elements of fluid-solid reactions
- Chapter 3. Reaction kinetics of fine particles
- Chapter 4. General approach to fluid-solid reaction analysis and modeling
- Chapter 5. Reactions of single nonporous solids
- Chapter 6. Reactions of single porous solids
- Chapter 7. Sohn’s law of fluid-solid reaction rates
- Chapter 8. Extension of basic analysis to complex systems
- Chapter 9. Reactions between solids proceeding through gaseous intermediates
- Chapter 10. Design of multiparticle fluid-solid reactors
- Chapter 11. Experimental techniques in fluid-solid reactions
- Index