
- 306 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Reproductive Technologies in Animals
About this book
Reproductive Technologies in Animals provides the most updated and comprehensive knowledge on the various aspects and applications of reproductive technologies in production animals as well as companion, wild, exotic, and laboratory animals and birds. The text synthesizes historical information and recent discoveries, while dealing with economical and geographical issues related to the implementation of the same technologies. It also presents the effects of reproductive technology implementation on animal welfare and the possible threat of pathogen transmission.Reproductive Technologies in Animals is an important resource for academics, researchers, professionals in public and private animal business, and students at the undergraduate and graduate levels, as it gives a full and detailed first-hand analysis of all species subjected to the use of reproductive technologies.
- Provides research from a team of scientists and researchers whose expertise spans all aspects of animal reproductive technologies
- Addresses the use of reproductive technologies in a wide range of animal species
- Offers a complete description and historical background for each species described
- Discusses successes and failure as well as future challenges in reproductive technologies
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Reproductive Technologies in Animals by Giorgio Presicce in PDF and/or ePUB format, as well as other popular books in Technik & Maschinenbau & Tierhaltung. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
Reproductive technologies in cattle
J. Richard Pursley and Jose Cibelli, Michigan State University, East Lansing, MI, United States
Abstract
In this chapter, a particular focus of attention has been given to the use of reproductive technologies for the manipulation of ovarian function and physiology. The implementation of fertility programs based on Ovsynch have been shown to be instrumental for enhancing reproductive performance and controlling calving interval. The adoption of such newly developed reproductive technologies has undoubtedly given proof to be valuable tools for increasing productivity in dairy cattle. In the second part of this chapter, more complex technologies such as cloning through somatic cell nuclear transfer will be discussed. They give the possibility to replicate the genome of highly valuable animals of superior genetics while underlining the limits, costs, and hurdles to be found in the various steps of the entire process. Finally, a vision will be given to the reader on the need to obtain more information on cellular reprogramming and cell plasticity, and on the new technical challenge regarding emerging assisted reproductive techniques, from the study and development of DNA markers for shortening generation interval and selection to the production of functional gametes from embryonic and primordial germ cells into sperm and eggs.
Keywords
Cattle; fertility programs; Ovsynch; cloning; emerging ARTs
1.1 Introduction
Reproductive technologies are critical for genetic modification and management efficiencies that directly impact sustainability of dairy and beef herds. These technologies provide ways to improve individual production and health traits as well as manipulate production cycles to maximize herd performance parameters.
This chapter describes current reproductive technologies from whole animal to cloning in cattle. Section 1 focuses on recent technologies that have transformed reproduction in cattle industries. Technologies that control ovarian function and timing of ovulation for the purpose of timed-artificial insemination (AI) are discussed in depth. These technologies succinctly control the time of ovulation and can improve fertility. The increases in reproductive efficiency from these technologies have had major positive impacts on profit of herds that utilize them. In addition, new electronic technologies that enhance detection of estrus and new ways to diagnose early pregnancies in cattle are discussed. Lastly, this section will introduce new ways to control ovarian development during ovarian stimulation with follicle-stimulating hormone (FSH).
Section 2 discusses bovine cloning and emerging reproductive technologies to improve cattle genomic parameters. It includes a brief description on the origins of cloning cattle, and the current techniques implemented commercially. We also discuss new technologies that, coupled with somatic cell nuclear transfer (SCNT), have the potential to exponentially increase genetic selection in a very short period of time.
Section 1: Increasing the efficiency of pregnancy production utilizing technologies that control ovarian function in cattle
1.1.1 Controlling ovarian development to enhance pregnancy production following artificial insemination
1.1.1.1 Physiological basis for ovarian manipulation of the estrous cycle
Technologies that manipulate ovarian structures in systematic ways are important for a number of reasons. The primary purpose of manipulating ovarian function is to synchronize timing of estrus and/or ovulation for preparation to AI. Additionally, synchronizing estrus and/or ovulation is important for recipients of embryos in addition to donor cows being prepared for superovulation or ovum pickup (OPU).
Follicles and corpora lutea (CL) growth and function during an estrous cycle are very well understood. Follicles grow in waves following estrus [1]. A cohort of antral follicles begins growing due to an FSH surge that occurs simultaneously with the luteinizing hormone (LH) surge at the onset of estrus. The largest of these follicles continues to grow and develop after the remainder of the follicles in the wave become atretic. This follicle has been termed the dominant follicle (DF) because the inhibin and estradiol that it produces inhibits the release of FSH, thus dominating the ovary as the only growing follicle until it either becomes atretic or, in the case of most second- or third-wave DFs, ovulates. FSH will surge once lack of inhibitory substances, inhibin and estradiol, are no longer being produced from the DF [2–4]. This natural FSH surge will induce a new follicular wave. Once the DF becomes atretic or ovulates a new wave of follicles starts the process again.
Acquisition of LH receptors in granulosa cells of the DF occurs when the DF deviates in growth from the other follicles in the wave [5]. This critical change in functionality to a follicle with LH receptors makes it possible to manipulate this follicle with LH, human chorionic gonadotropin (hCG), or an exogenously induced LH surge using gonadotropin-releasing hormone (GnRH). Understanding this mechanism opened doors to novel ways to manipulate ovarian function and control time to estrus and ovulation. For example, gaining a greater understanding of these concepts led to the discovery of Ovsynch (Fig. 1.1 [6]).

Historically, four types of hormones have been utilized to manipulate ovarian function: GnRH type products including the direct impacts of LH or hCG, prostaglandin F2-alpha (PG), progesterone (P4), and pharmaceutical estrogens. The earliest technology derived from one of these hormones was the systematic use of PG to induce and schedule estrus in cattle [3,7]. This technology enhanced the percentage of cows detected in estrus and provided a management tool to organize labor more efficiently.
1.1.1.2 Importance of inducing a new follicular wave
The most important step in assuring success of timed-AI technologies is the induction of a new follicular wave. It is a critical step in manipulating ovarian development to control time of ovulation to allow for timed-AI. A new follicular wave can be pharmacologically induced following ovulation with an endogenous or exogenous surge of LH, estrogen-induced atresia of functional follicles, or ablation of functional follicles with ultrasound-guided aspiration [8].
Asynchrony of follicle development relative to luteolysis and induction of ovulation is likely to occur if a new follicular wave is not induced in timed-AI technologies. DFs can become atretic prior to PG induced luteolysis. This would result in an untimely new wave of follicles near the time of the final induction of ovulation. Thus follicles from this untimely new wave may lack sufficient maturity to respond to the final LH surge and ovulate. Delayed time to estrus and ovulation would occur 3–4 days later.
GnRH products induce an LH surge from the anterior pituitary. Functional DFs generally ovulate approximately 28 hours following an LH surge even in the presence of progesterone. An FSH surge occurs at the same time as the LH surge, and stimulates a new wave of follicular development. Numbers of follicles in this new wave are positively associated with circulating levels of anti-Müllerian hormone [9].
1.1.2 Ovsynch technologies
Ovsynch technologies utilize GnRH and PG in timely fashions to synchronize ovulation during a period of low progesterone imitating as closely as possible the physiological environment of a natural estrus [6]. Ovsynch technologies control follicle and CL development in order to synchronize ovulation amongst groups of cattle for fixed timed-AI [10,11]. Most countries have approved GnRH and PG products available for use. GnRH products are decapeptides that may or may not have a hydrophobic peptide for a longer half-life [12]. Most PG products utilized in Ovsynch technologies contain either cloprostenol sodium or dinoprost tromethamine. Ovsynch-based technologies generally include Ovsynch, Double Ovsynch, G6G, and Presynch/Ovsynch protocols. Ovsynch may include the use of a progesterone-releasing device during the period between the GnRH and PG.
1.1.2.1 The basis for development of Ovsynch-based technologies
Embryonic survival continues to be a critical problem in lactating dairy cows, limiting thus profitability and sustainability of dairy farms [13]. The inability of the lactating dairy cow to foster complete embryonic and fetal development is escalating due to greater genetic pressure on milk production. Pregnancies per AI at 32 days following a detected estrus are approximately 30% in Holstein cows compared to 60% in virgin dairy heifers with similar genetic makeup [14].
Remarkable changes in follicular dynamics occur in dairy cattle during transition from nulliparous to primiparous and multiparous. Size of the ovulatory follicle, length of follicular dominance, number of double ovulations (twinning rates), and ovarian cysts [15] are greater in cows versus nulliparous heifers. Nulliparous heifers have approximately double the progesterone concentrations during mid-luteal phase of the estrous cycle compared to cows. The considerable decrease in fertility and increase in twinning rates transitioning from nulliparous heifer to cow negatively affects farm profit. “Fertility” programs (Fig. 1.2) were developed in the past 15 years to increase pregnancies per AI and reduce the chance for twinning compared to Ovsynch alone or natural estrus [16–18]. Fertility programs utilize pre-Ovsynch hormonal manipulations to increase levels of progesterone and manipulate the age and size of ovulatory follicles in cows treated with Ovsynch [19].
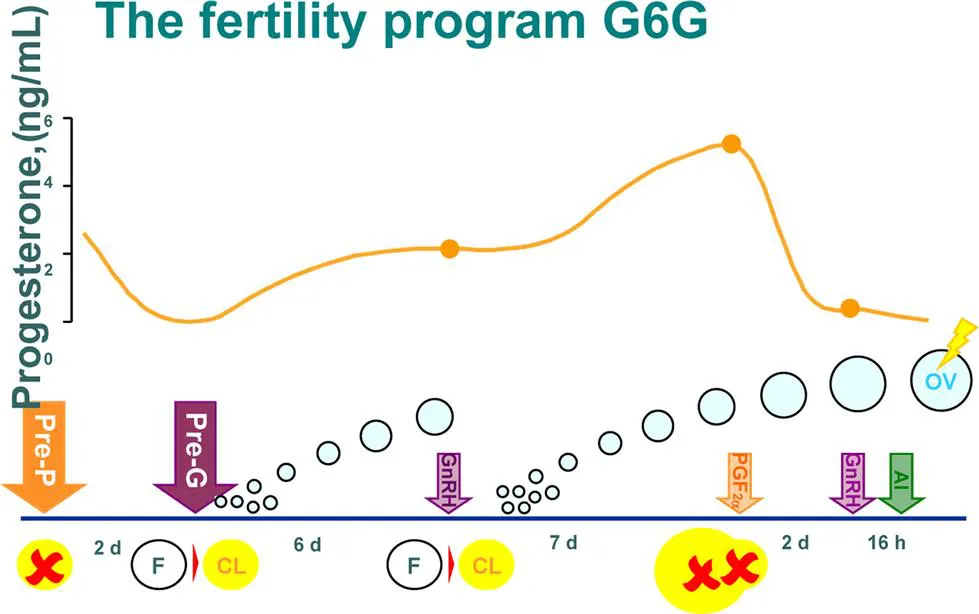
1.1.2.2 First gonadotropin-releasing hormone of Ovsynch
Fertility program technologies in dairy cows are designed to “presynchronize,” so Ovsynch can be implemented on day 6 or 7 of the estrou...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- List of contributors
- Chapter 1. Reproductive technologies in cattle
- Chapter 2. Assisted reproductive biotechnologies in the horse
- Chapter 3. Reproductive technologies in sheep
- Chapter 4. Reproductive technologies in goats
- Chapter 5. Reproductive technologies in swine
- Chapter 6. Reproductive technologies in the buffalo (Bubalus bubalis)
- Chapter 7. Reproductive technologies for the conservation of wildlife and endangered species
- Chapter 8. Reproductive technologies in camelids
- Chapter 9. Reproductive technologies in companion animals
- Chapter 10. Reproductive technologies in laboratory animals
- Chapter 11. Standard and innovative reproductive biotechnologies for the development of finfish farming
- Chapter 12. Assisted reproductive technologies in nonhuman primates
- Chapter 13. Reproductive technologies in avian species
- Chapter 14. Reproductive technologies in the honeybee (Apis mellifera)
- Chapter 15. Spermatogonial stem cells: from mouse to dairy goats
- Chapter 16. Reproductive technologies and pathogen transmission
- Chapter 17. Reproductive technologies and animal welfare
- Index