
eBook - ePub
Recycling of Polyethylene Terephthalate Bottles
- 212 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Recycling of Polyethylene Terephthalate Bottles
About this book
Recycling of Polyethylene Terephthalate Bottles provides an overview of PET chemistry, highlighting the main degradation, depolymerization processes and pathways of PET, along with the applications of recycled monomers derived from PET waste. The latest methodologies of recycling and feedstock recovery are covered, providing critical foundational information. In addition, the book discusses a range of established methods of polymer recycling, with an emphasis on real world industrial case studies and the latest academic research. Users will find in-depth lifecycle and cost analysis of each waste management method, comparing the suitability and feasibility of each to support the decision -making process.
Polyethylene Terephthalate (PET) is the most recycled plastic in the world, but still represents a significant amount of landfill waste. This book presents an update on new regulations, providing recommendations for new opportunities in this area, including new processing methods and applications for recycled PET.
- Features a comprehensive introduction to the waste management of PET bottles, from regulatory concerns, to the range of different methods of materials recovery
- Enables practitioners to choose the most efficient and effective waste management process
- Includes detailed lifecycle and cost analysis information
- Compares traditional thermal recycling methods with more recently developed monomer recovery and chemical recycling methods
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Recycling of Polyethylene Terephthalate Bottles by Sabu Thomas, Ajay Vasudeo Rane, Krishnan Kanny, Abitha VK, Martin George Thomas, Sabu Thomas,Ajay Vasudeo Rane,Krishnan Kanny,Abitha VK,Martin George Thomas in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Engineering General. We have over one million books available in our catalogue for you to explore.
Information
1
PET Chemistry
Suranjana Mandal and Ayan Dey, Department of Plastic and Polymer Engineering, Maharashtra Institute of Technology, Aurangabad, Maharashtra, India
Abstract
Polyethylene terephthalate (PET) is popular for its widespread use from thermoplastic product to fiber. However, the end property, melting behavior, and processing technology for PET depend on the chemistry of the reactant, catalyst, and other additives used here. This chapter not only provides insight of chemistry of the polymer but also describes the structure–property relationship for the same. The reaction and mechanisms involved during PET synthesis are briefly discussed here.
Keywords
Polyethylene terephthalate; thermoplastic; fiber; chemistry; structure-property relationship
1.1 Introduction
Polyethylene terephthalate or poly(ethylene terephthalate), abbreviated as PET, it is one of the most abundantly available semicrystalline thermoplastic resin and it is the only fiber and film forming saturated polymer with commercial importance [1].
This is the commercially popular polymer used in fibers for clothing, liquid, and food container. In addition PET has good mechanical property (such as tensile and tear strength), heat sealability, excellent barrier property against oxygen, carbon dioxide, anhydride, aroma compound, etc. [2]. This polymeric resin is also used in the production of thermoformed structure as well as used in combination of glass fiber as composite.
W.H. Carothers, an American Chemist and inventor of Nylon first synthesized thermoplastic polyesters from adipic acid and ethylene glycol (EG), in 1929. In 1941, high molecular weight polyesters based on terephthalic acid (TPA) was synthesized, which became a product of industrial interest. J.R. Whinfield and J.T. Dickson of Calico Printers Association in England, invented poly(ethylene terephthalate) fiber (Terylene, Dacron, Lavsan) and also film (Melinex, Mylar) and moulding material which becomes promising candidate for producing blow moulded bottles.
In the 1950s, Du Pont and Imperial Chemical Industries, Ltd., began manufacture of fiber melt–spun synthetic polyester products. Yet it was not until the mid-1960s that thermoplastic polyesters were employed as construction materials. These were fast-crystallizing grades of PET containing additives that gave uniform and controlled morphology.
A short time later, copolyesters were developed with enhanced properties. PET containers formed by stretch blow molding were introduced in the United States by Du Pont in the mid-1970s. Since that time, the market for PET has grown at 10% per year, with initial growth spawned by direct replacement of glass and metal, as well as the introduction of new products such as heat-set containers.
Today, PET is used to produce a variety of products, including filament and staple fiber, film, tire cord, technical yarns, and packaging resins. PET may be produced from EG with either purified terephthalic acid (PTA) or dimethyl terephthalate (DMT) in presence of a metal ion (catalyst). Till 1960, DMT was preferred as feedstock for PET manufactures because of the fact that ester could generally be made in more pure form than acid. Later, Amoco developed high purity terephthalic acid process, which becomes the preferred feedstock for the production of PET. Advantage of using highly PTA eliminates the need of recycling methanol, rapid esterification of the prepolymer and initial step of esterification process (when DMT is in use as feedstock).
1.2 Physical and Chemical Properties
PET, a type of thermoplastic engineering polyester possesses excellent mechanical, electrical, and thermal properties. Moreover it has very good chemical resistance, transparency, dimensional stability, and low moisture absorption. The important properties are enlisted below [3,4]:
- 1. Transparent and light weight
- 2. Extremely low moisture diffusion through the polymer matrix
- 3. Exceptional dimensional stability
- 4. Excellent electrical property
- 5. Excellent chemical resistance
- 6. Good environmental stress crack resistance
- 7. Very good resistance to thermal aging
- 8. Very low creep at wide temperature range
- 9. Very good color stability
- 10. Excellent wear resistance
- 11. Low flavor adsorption
- 12. Better aesthetic appearance (compared to other polymers)
- 13. Economic availability and easy process ability
- 14. Excellent recyclability
Higher ordered regularities in chemical and geometric architecture of the polymer result in crystalizability of the PET matrix. Either it is semicrystalline or amorphous in nature. PET with high crystallinity possesses higher glass transition temperature compared to amorphous PET along with mechanical properties like higher modulus, toughness, stiffness, tensile strength, hardness, and more solvent resistance. The impact strength of the material becomes inferior with the increase in crystallinity [5].
Fig. 1.1 shows the impact of the ester group concentration on the melting point of different linear polyester. The structures of homologous polyesters are shown in Table 1.1. For the linear aromatic polyesters, melting point decreases with the decrease in the concentration of ester groups. However, the variation of melting points with the increasing number of methylene group in between two ester linkages present in the backbone is of conspicuous nature.

Table 1.1
| Series | Structure of Repeat Units |
|---|---|
1. Poly(alkylene biphenyl-4,4'-dicarboxylate) | 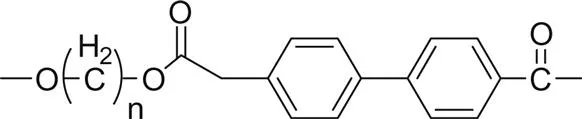 |
2. Poly(p-phenylene alkandioate) | 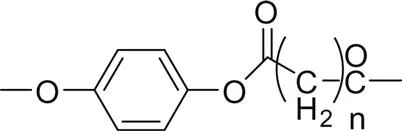 |
3. Poly(alkylene terephthalate) | 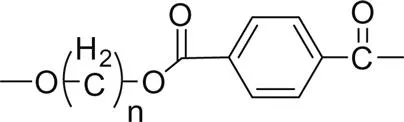 |
4. Poly(alkylene succinate) | 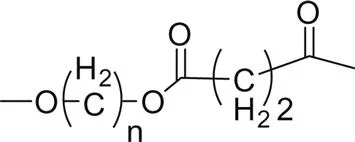 |
5. Poly(decamethylene alkane dioate) | 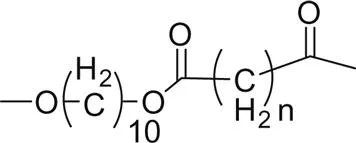 |
6. Poly(alkylene adipate) | 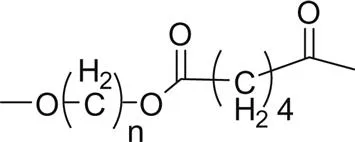 |
To resolve the mystery, the data of melting points of polyesters available in the literature have been plotted separately for even and odd numbered of carbon atoms. The variation of the melting point has been found to be same for both of them with increasing number of methylene groups in between two ester linkages. The melting point has been found to decrease exponentially and attains a plateau when number of methylene groups reaches to six. However, it can also be said that decrease in concentration of ester group causes a slight increase in the melting point.
The polyesters are illustrated in Table 1.2. With the increase in methylene groups in the repeating unit, the polymer behaves like linear polyethylene (polymethylene). Thus the melting points for five polyesters among the classes available are seen to converge towards that of the polymethylene. In the case of the sixth class, the poly(alkylene adipates) behaves differently. It is interestingly observed that the melting point for polymers with off numbered methylene groups in the aliphatic moiety is significantly lower than the melting point for the polymers composed of even number of m...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- List of Contributors
- 1. PET Chemistry
- 2. Regulations on Recycling PET Bottles
- 3. Materials Recovery, Direct Reuse and Incineration of PET Bottles
- 4. Chemical Depolymerization of PET Bottles via Glycolysis
- 5. Depolymerization of PET Bottle via Methanolysis and Hydrolysis
- 6. Chemical Depolymerization of PET Bottles via Ammonolysis and Aminolysis
- 7. Chemical Depolymerization of PET Bottles via Combined Chemolysis Methods
- 8. Life Cycle Assessment (LCA) of PET Bottles
- 9. Applications of Waste Poly(Ethylene Terephthalate) Bottles
- Index
