
Intermediate Temperature Solid Oxide Fuel Cells
Electrolytes, Electrodes and Interconnects
- 516 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Intermediate Temperature Solid Oxide Fuel Cells
Electrolytes, Electrodes and Interconnects
About this book
Intermediate Temperature Solid Oxide Fuel Cells: Electrolytes, Electrodes and Interconnects introduces the fundamental principles of intermediate solid oxide fuel cells technology. It provides the reader with a broad understanding and practical knowledge of the electrodes, pyrochlore/perovskite/oxide electrolytes and interconnects which form the backbone of the Solid Oxide Fuel Cell (SOFC) unit. Opening with an introduction to the thermodynamics, physiochemical and electrochemical behavior of Solid Oxide Fuel Cells (SOFC), the book also discusses specific materials, including low temperature brownmillerites and aurivillius electrolytes, as well as pyrochlore interconnects.This book analyzes the basic concepts, providing cutting-edge information for both researchers and students. It is a complete reference for Intermediate Solid Oxide Fuel Cells technology that will be a vital resource for those working in materials science, fuel cells and solid state chemistry.- Provides a single source of information on glass, electrolytes, interconnects, vanadates, pyrochlores and perovskite SOFC- Includes illustrations that provide a clear visual explanation of concepts being discussed- Progresses from a discussion of basic concepts that will enable readers to easily comprehend the subject matter
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Understanding intermediate temperature solid oxide fuel cells
Abstract
Keywords
1.1 Introduction to fuel cells
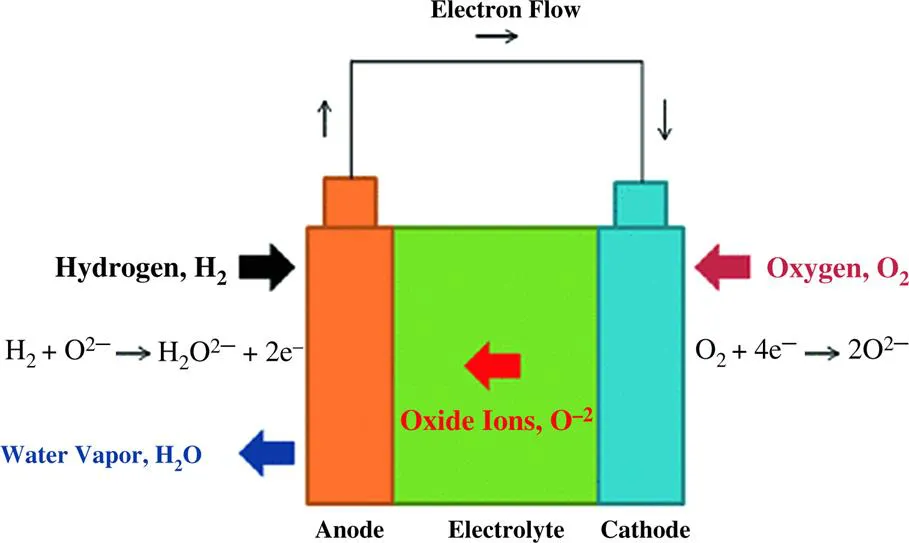
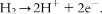
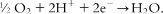

1.2 Operating temperature of fuel cells

Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Dedication
- List of contributors
- About the editor
- Foreword
- Acknowledgments
- Chapter 1. Understanding intermediate temperature solid oxide fuel cells
- Chapter 2. Thermodynamics, polarizations, and intermediate temperature solid oxide fuel cell performance
- Chapter 3. Brownmillerite and Aurivillius electrolytes for intermediate temperature solid oxide fuel cell
- Chapter 4. Proton-conducting electrolyte materials
- Chapter 5. Enhancing the ionic conductivity in the ceria-based electrolytes for intermediate temperature solid oxide fuel cells
- Chapter 6. Cermets as anode materials
- Chapter 7. Progress in perovskite anodes for intermediate-temperature solid oxide fuel cells
- Chapter 8. Cathode materials for proton-conducting solid oxide fuel cells
- Chapter 9. Perovskite and layered oxide materials for intermediate temperature solid oxide fuel cells
- Chapter 10. Misfit-layered Ca-cobaltite–based cathodes for intermediate-temperature solid oxide fuel cell
- Chapter 11. Stacking designs and sealing principles for IT-solid oxide fuel cell
- Chapter 12. Interaction of glass-ceramic sealants with solid oxide fuel cell components: thermo-mechanical analysis
- Chapter 13. Intermediate-temperature solid oxide fuel cell fueled by biofuels
- Index