![]()
Anatomy and Physiology of the Peritoneum
The peritoneum, which can be used as a dialysis membrane, consists of the mesothelium and the underlying interstitial tissue that contains the microvessels. In adults, the mesothelial surface area averages at 0.55 m2 by CT scanning, which is about one third of the skin surface. Solutes and water are transported from peritoneal capillaries through the interstitium and mesothelium to the dialysate-filled peritoneal cavity and vice versa. The mesothelium offers no resistance to transport; the possible role of the interstitium will be discussed later.
Solute Transport
The number of peritoneal capillaries perfused is the most important determinant of solute transport, not the peritoneal blood flow. Endothelial transport occurs through an abundance of transendothelial pores and interendothelial water channels. Small solutes like urea, creatinine, and glucose traverse the endothelium by diffusion through small pores that have radii of about 40 Å. Even β2-microglobulin, which has a radius of 17 Å, can pass the small pores without hindrance. Consequently, the differences in the transport of various small solutes are dependent on differences in their diffusion velocity (molecular weight [MW] and shape) and not on the intrinsic permeability of the peritoneal membrane. The transperitoneal concentration gradient of a solute is the 3rd determinant of diffusion. It decreases during a dialysis dwell due to saturation of the dialysate. Consequently, the dialysate/plasma ratio, which equals 0 in the beginning of a dialysis exchange, increases during the dwell and finally reaches 1.0, at which time no effective diffusion occurs. For urea (MW 60 Da), this equilibrium is approached after 4 h in many patients. In this situation, the drained dialysate volume is the only determinant for the removal of urea from the body. Equilibrium for creatinine (MW 113 Da) takes longer. Diffusion also occurs for intraperitoneally administered osmotic agents like glucose (MW 180 Da). The absorption of glucose averages 60% after 4 h, which has a marked effect on its osmotic activity during a dwell.
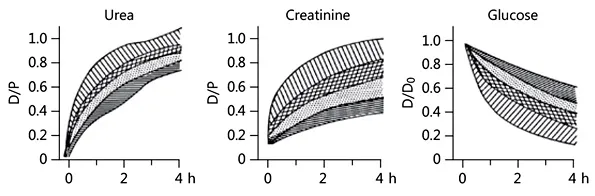
Fig. 1. The various solute transport categories. Note that the high creatinine transporters have the fastest disappearance of glucose. D/P, dialysate/plasma concentration ratio; D/D0, dialysate concentration at a time/dialysate concentration before inflow.
Marked differences in peritoneal solute transport are present among patients, dependent on the magnitude of the effective surface area and thus the number of microvessels perfused. The dialysate/plasma ratio of creatinine after a dwell time of 4 h can range between 0.34 and 1.00 (mean 0.65). Based on mean values and standard deviations of a stable peritoneal dialysis (PD) population, patients have been divided into high, low, and average (high and low average) transporters, as shown in Figure 1.
The above terminology is very confusing, because high transporters usually have low ultrafiltration (UF) rates, caused by a high glucose absorption, which leads to a rapid disappearance of the osmotic gradient. A low ultrafiltered volume results in impaired removal of urea and other small molecules. Because of this phenomenon “high” is better replaced by “fast” and “low” by “slow.” Fast transporters have an increased risk of overhydration. A meta-analysis reported that patients with a fast transport status had higher mortality than the others. However, this was only the case for those treated with CAPD (continuous ambulatory PD) and glucose as osmotic agent. The CAPD scheme consists of 3 short dwells (4–6 h) and 1 long dwell (8–10 h). Especially during the long dwell, fluid removal can be low or absent. Fast-transport patients treated with APD (automated PD) in whom a long dwell can be avoided had no increased mortality. Survival was also not impaired in patients treated with icodextrin (a poorly absorbed high-MW osmotic agent) for the long dwell. Long-term PD is often associated with the development of fast small-solute transport rates, pointing to a large effective surface area due to diabetiform neoangiogenesis. A fast transport status can also occur early in the time course of PD, for instance in patients with inflammation due to marked vascular comorbidity as well as by the development of endothelial-mesenchymal transition (EMT) of mesothelial cells. Clinically, this is characterized by fast solute transport, high effluent concentrations of the mesothelial cell marker cancer antigen (CA)-125, and also by high effluent levels of the vascular endothelial growth factor. The latter is an important mediator of diabetic retinopathy. Some have claimed that EMT is an early stage of long-term structural peritoneal abnormalities but without proof. The functional changes of EMT in PD are a transitory phenomenon.
Table 1. Pressure gradients
| In dialysate filled peritoneal cavity | Pressure gradient |
Hydrostatic pressure, mm Hg | 8 (recumbent) | 17 |
Colloid osmotic pressure, mm Hg | 0.1 | –21 |
Osmolality, mosm/kg H2O | 347 (glucose 1.36%)
486 (glucose 3.86%) | Dependent on the RC |
Initial crystalloid osmotic pressure gradient, mm Hg for RC = 0.03 | Glucose 1.36%
Glucose 3.86% | 24
105 |
RC, reflection coefficient. |
Fluid Transport
Removal of excess fluid from the body is probably the most important goal of PD. The transport of water and dissolved solutes to and from the peritoneal cavity is determined by hydrostatic and osmotic pressure gradients and also by lymphatic drainage. The pressure gradients that influence transcapillary UF are summarized in Table 1. The effectiveness of the crystalloid osmotic pressure is determined by the osmolality of the solution and its capability to induce a pressure gradient across a membrane. The latter is dependent on the reflection coefficient. This parameter can range between 0 and 1.0. The solute passes the membrane without any hindrance and has no osmotic effect when 0. A reflection coefficient of 1.0 means that the membrane is semipermeable, i.e., that the solute cannot pass, but water can. In this situation, 1 mosm/kg H2O induces an osmotic pressure of 19.3 mm Hg. The reflection coefficient of glucose across the peritoneum averages at 0.03, meaning that large osmolality gradients are required to induce a crystalloid osmotic pressure gradient. These gradients decrease during a dwell due to absorption of glucose. The glucose absorption explains why the intraperitoneal volume increases initially and decreases thereafter.
Replacement of glucose by the glucose polymer icodextrin (see Chapter 9 Peritoneal Dialysis Solutions) causes sustained UF, because it hardly diffuses from the peritoneal cavity and creates a colloid osmotic pressure gradient.
The paradoxical observation that glucose causes UF despite its low reflection coefficient is explained by the presence of the water channel aquaporin-1 (AQP-1) in capillary and venular peritoneal endothelial cells. Solutes cannot traverse AQP-1, meaning that the reflection coefficient of glucose to this channel equals 1.0. Therefore, AQP-1 induces free water transport (FWT). The initial contribution of FWT to total transcapillary UF averages 40%. FWT explains the decrease in dialysate Na+ (sodium sieving), a dilutional phenomenon that occurs in the initial phase of a dwell with the highest glucose concentration. Icodextrin only induces fluid transport through the small pores and thus no sodium sieving.
Assessment of lymphatic absorption from the peritoneal cavity and tissues requires the use of a macromolecular marker, where diffusion can be neglected. The marker can be given intravenously or intraperitoneally. In the first case, the appearance rate in the dialysate is measured, and in the other case the disappearance rate from the dialysate. The values obtained with the appearance rates average 0.2 mL/min, those with the disappearance rate 1.5 mL/min. The difference can partly be explained by the absence of a steady state for the appearance rate and by transmesothelial transport of the intraperitoneal marker to the interstitium. Clinical research has mainly been done using the peritoneal clearance (disappearance rate) of neutral dextran. Consequently, the method gives an assessment of overall lymphatic uptake, i.e., from the peritoneal cavity and from the interstitial tissues. Results of those studies showed that the effective lymphatic absorption rate is influenced by the intraperitoneal pressure but hardly by posture. Also, the duration of PD has no effect. A small minority of patients have a high value already in the beginning of PD. These patients often have otherwise unexplained UF failure (UFF).
UFF is by far the most important complication of long-term PD, because it often leads to overhydration. The two must clearly be distinguished, because overhydration can also develop because of a decrease in residual urine production, excessive fluid intake, an inadequate dialysis prescription, or peritoneal leakage. Therefore, the diagnosis UFF should be based on a standardized dialysis exchange. According to the 3 × 4 rule, UFF is present when net UF after a 4-h dwell is <400 mL using a dialysis solution based on 3.86%/4.25% glucose. Early UFF is often not a big risk for overhydration, because most patients will still have urine production. Acute peritonitis causes temporary UFF, because the effective peritoneal surface area is enlarged due to the effect of inflammation on the number of peritoneal microvessels perfused, which leads to a rapid disappearance of the crystalloid osmotic gradient. Late UFF is a serious complication, because it is associated with structural peritoneal abnormalities and often leads to overhydration. Late UFF is present in about 20% of patients treated with PD for >2 years. Enlargement of the vascular peritoneal surface area is the most frequent cause, because it leads to a rapid disappearance of the crystalloid osmotic pressure gradient. Long-term exposure to the extremely high glucose concentrations in the dialysis solutions causes diabetiform neoangiogenesis and vasculopathy. The latter is likely to reduce the filtration pressure and thereby ultrafiltrat...