Advances in New Drugs for Diabetes Treatment: GLP-1 Mimetics and DPP-4 Inhibitors
INTRODUCTION
The prevalence of obesity and type 2 diabetes mellitus (T2DM) are evolving globally at an alarming rate in the 21st century as a result of overnutrition, an
ageing population, lack of exercise, and increased migration of susceptible patients. The International Diabetes Federation estimated in 2025 that 380 million adults worldwide had diabetes [1]. However, the global statistics of diabetes mellitus in year 2013 has indicated already, about 382 million people had this disease worldwide [2]. In year 2012 and 2013 diabetes resulted in mortality of 1.5-5.1 million people per year, making it the 8th leading cause of death in the world [2]. And the World Health Organization (WHO) has estimated the prevalence of DM in the USA in 2025 is 11.2% [3]. In addition, an average age of onset of diabetes is about 42.5 years and the economic cost of diabetes seems to have a significant increased worldwide. The primary aim of managing T2DM is to delay, or even prevent the complications of the disease by achieving good glycaemic control. Therefore, the need for new effective and long-lasting anti-diabetic drugs is urgent.
Increased knowledge of pathophysiology of T2DM has contributed to the development of novel treatments, such as glucagon-like peptide-1 (GLP-1) mimetics and dipeptidyl peptidase-4 (DPP-4) inhibitors. GLP-1 agonists mimic the effect of incretin, whereas DPP-4 inhibitors prevent the inactivation of the endogenously released hormone. Both agents offer an effective alternative to the currently available hypoglycaemic drugs. And they may also hold a potential role in preventing obesity and diabetes as compared to other weight loss agents. However, further evaluation is needed to confirm their clinical roles and safety. The aim of this review is to summarize the background of the incretin, and explore the roles and side effects of the GLP-1 agonists and DPP-4 inhibitors. In addition, their weight-lowering properties will be summarized specially.
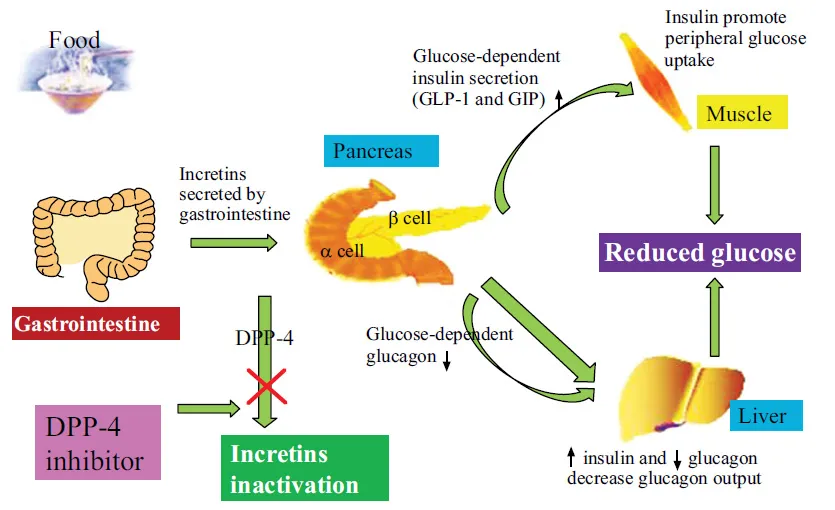
INCRETINS
Incretins are a group of metabolic hormones that stimulate a decrease in blood glucose levels (in Fig. 1). And they are gut-derived peptides secreted in response to meals. The major incretins are GLP-1 and glucose dependent insulinotropic peptide (GIP) and they account for approximately 90% of the incretin activity [4]. Both GLP-1 and GIP are rapidly inactivated by the enzyme DPP-4. The GLP-1is produced by L-cells in the distal gut, and the GIP is produced by duodenal K-cells in response to ingested nutrients [5]. The incretins are secreted into the circulation within minutes in response to a meal and up on release they bind to specific G-protein coupled receptors present on cells and other target tissues [6]. GLP-1 is secreted in greater concentrations than GIP and is considered more physiologically relevant in humans [7]. In β-cells, GLP-1 enhances glucose-dependent insulin secretion, increases insulin synthesis, and in animals stimulates β-cell proliferation and inhibits apoptosis [5]. GLP-1 also reduces glucose concentrations through inhibition of pancreatic α-cell glucagon secretion and indirectly via inhibition of gastric emptying and appetite [8, 9]. In addition to slowing gastric emptying, GLP-1 may also decrease intestinal lymph flow, triglyceride absorption, and apolipoprotein synthesis adding to a complex combination of mechanisms that may limit the release of triglycerides into the circulation after lipid-containing meals [10].
GLP-1 levels are significantly decreased in T2DM (approximately 50% compared to healthy individuals) [11, 12]. Therefore, the incretin-based therapy has emerged as a strategy in managing T2DM, primarily because they generally do not cause hypoglycemia and possess weight-neutral or weight losing properties. And the efficacy of these agents is more or less similar to commonly used drugs metformin and sulfonylureas. Early GLP-1 therapy was suggested to preserve β -cell function in subjects with impaired glucose tolerance (IGT) or mild T2DM [13]. Interestingly, a recent meta-analysis suggested that Asians may have better response to these incretin-based therapies [14].
THE ROLES OF THE GLP-1 AGONISTS
GLP-1 is a 30-amino acid peptide produced in the intestinal epithelial L-cells of the distal ileum and colon by differential processing of the proglucagon gene from the prohormone convertase PC1/3 [15]. GLP-1 binds to GLP-1 receptor in the cell membrane of the pancreatic islets [16]. And it exerts its action on β-cell proliferation and survival by phosphatidylinositol-3 kinase (PI3-K) and protein kinase B (PKB/Akt), p38 mitogen-activated protein kinase (MAPK) and protein kinase Cζ pathways [17, 18].
GLP-1 amide is not very useful for treatment of type 2 diabetes mellitus, since it must be administered by continuous subcutaneous infusion. Therefore, several long-lasting analogs have been developed. So far, exenatide and liraglutide have been approved for use widely. These agents have benefits of a lower risk of hypoglycemia, and having a potential for weight reduction. Moreover, these incretin- based therapies also exert anti-inflammatory activity [19]. However, the main disadvantage of these GLP-1 analogs is they must be administered by subcutaneous injection. Exenatide is administered twice daily or once weekly as a microsphere preparation, and liraglutide is a once-daily formulation.
Exenatide
Exenatide, the first FDA approved GLP-1mimetic,is the synthetic form of the naturally occurring exendin-4, a 39-amino-acid peptide hormone secreted by the salivary glands of the venomous lizard Heloderma suspectum [20]. It has only 53% homology to the human GLP-1 amino acid sequence. So it is relatively more resistant to DPP-4, reaching a maximum level approximately 2 hours following subcutaneous injection [21]. Exenatide plays many of the actions of GLP-1, such as enhancement of glucose induced insulin secretion, inhibition of glucagon release, reduction of fasting and postprandial glucose, delay of gastric emptying, inhibition of appetite and induction of weight loss [21 - 24]. Moreover, exenatide was associated with significant improvement in multiple cardiovascular risk factors including systolic and diastolic blood pressure, fasting triglycerides, as well as total, LDL- and HDL-cholesterol [25]. In addition, the anti-atherosclerotic effect of exenatide was associating with inhibition of inflammatory responses of atherosclerotic plaque macrophages [26]. In a word, exenatide played important roles in obese and T2DM in a large number of clinical trials.
One hundred fifty two obese (BMI: 39.6 ± 7.0 kg/m2) individuals were randomized to receive either exenatide or placebo, along with lifestyle modification for 24 weeks [27]. Exenatide treated individuals lost 5.1 ± 0.5 kg from baseline vs. 1.6 ± 0.5 kg in the placebo group. An important percentage of individuals with prediabetes returned to normal glucose after the end of the period. Therefore, exenatide therapy in addition to lifestyle modification is a promising therapeutic approach for obese prediabetic individuals. In another non randomized study, 105 individuals with IGT and/or IFG were treated with: (1) Lifestyle modification only ;(2) Pioglitazone 15mg daily and metformin 850mg daily; and (3) A triple combination of pioglitazone, metformin and exenatide [28]. In the pioglitazone and metformin group, insulin sensitivity and β-cell function improved by 42% and 50% respectively, while 14% of the individuals with IGT and 36% of the individuals with IFG reverted to NGT. Interestingly, in the triple therapy group, a robust 109% improvement in β-cell function and a 52% increased in insulin sensitivity was observed, while 59% of the individuals with IGT and 56% of the individuals with IFG reverted to NGT. In addition, a 24-week prospective randomized outpatient clinical trial explored the possible role of exenatide and metformin, alone or in combination, in 60 overweight/obese women with polycystic ovary syndrome [29]. ...