
Enzymes Involved in Glycolysis, Fatty Acid and Amino Acid Biosynthesis: Active Site Mechanisms and Inhibition
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Enzymes Involved in Glycolysis, Fatty Acid and Amino Acid Biosynthesis: Active Site Mechanisms and Inhibition
About this book
Multidisciplinary research involving crystallography, kinetic studies, molecular docking, genetics and other techniques in biochemistry has yielded a wealth of knowledge about the reaction mechanisms in cellular processes. This knowledge has allowed researchers to understand, in a better way, the normal functioning of the cell process, which is used as a reference point for learning about and preventing or correcting pathologies that cause diseases.
This enzymology reference is a thorough compendium about reaction mechanisms occurring between the major enzymes related to the biosynthetic pathways of 3 important types of biological compounds – 6-carbon carbohydrates, fatty acids and amino acids – and their substrates, cofactors and residues. Readers will gain an understanding of the interaction between substrates or ligands with specific amino acid residues in biosynthetic enzymes. This understanding builds a foundation for learning about the biochemistry of different inhibitors used in the treatment of several diseases such as cancer, infectious diseases, and metabolic syndrome alterations such as diabetes and obesity.
Enzymes covered in the book include aldolases, isomerases, kinases, mutases, synthases, dehydrogenases, reductases, transferases, hydrolases, lyases among others, all of which are wide spread in biochemical transformations.
This reference, with its insights on common biochemical enzymes serves as a handy guide for students, researchers and professionals involved academia or industry related to pharmaceutical development, healthcare, food chemistry and other disciplines.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Aminoacid Biosynthesis
Marco Brito-Arias*
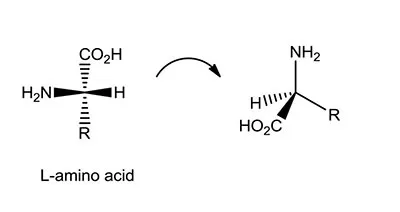
Tetrahedral projection of amino acids.
1. GLYCINE BIOSYNTHESIS
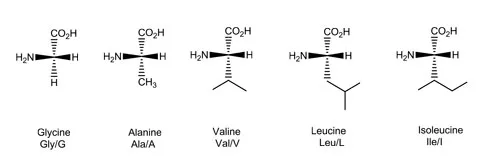



Classification and structure of amino acids.

General scheme of glycine biosynthetic precursors.


General scheme of glycine biosynthesis from serine in the presence of tetrahydrofolate and pyridoxal phosphate cofactors.


Proposed reaction mechanism for glycine formation involving PLP and H4PteGlu.
1.1. Serine Hydroxyl Methyl Transferase (SHMT)
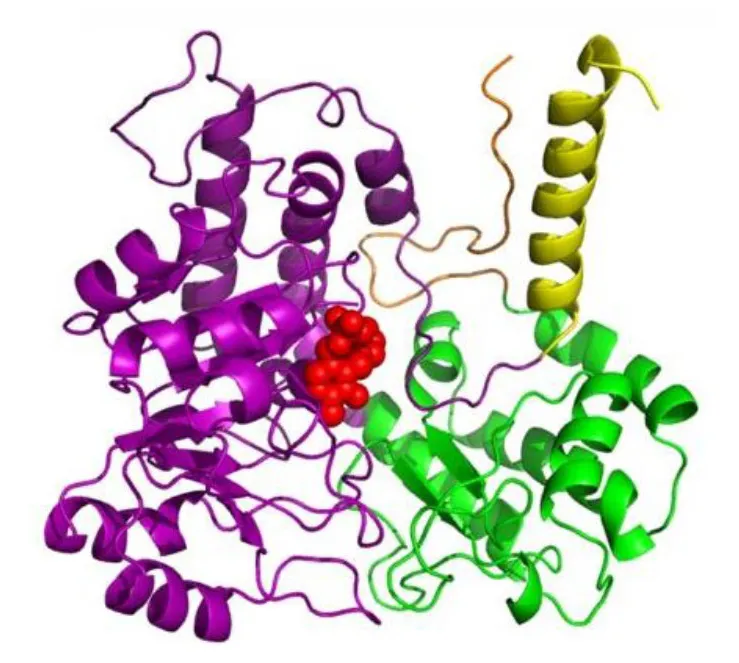
The ribbon structure of G monomer SHMT from Methanocaidococcus jannaschii G monomer showing the catalytic domain in purple, the C-terminal in green, and PLP as spheres in red (PDB: 4BHD).
Table of contents
- Welcome
- Table of Content
- Title
- BENTHAM SCIENCE PUBLISHERS LTD.
- PREFACE
- ACKNOWLEDGEMENTS
- Glycolysis
- Citric Acid Cycle (Krebs)
- Fatty Acid Biosynthesis
- Aminoacid Biosynthesis
- References