
eBook - ePub
Advances in Chromatography
Volume 58
Nelu Grinberg, Peter W. Carr, Nelu Grinberg, Peter W. Carr
This is a test
Share book
- 198 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Advances in Chromatography
Volume 58
Nelu Grinberg, Peter W. Carr, Nelu Grinberg, Peter W. Carr
Book details
Book preview
Table of contents
Citations
About This Book
For six decades, scientists and researchers have relied on the Advances in Chromatography series for the most up-to date information on a wide range of developments in chromatographic methods and applications. The clear presentation of topics and vivid illustrations for which this series has become known makes the material accessible and engaging to analytical, biochemical, organic, polymer, and pharmaceutical chemists at all levels of technical skill.
Key Features:
-
- Discusses the basic concepts of affinity chromatography and examines recent developments in this method and related supramolecular separation methods.
-
- Outlines the different types of gradient stationary phases and how they have been used in and benefited the field of separation science.
-
- Reviews recent trends in detectors for GC, focusing on those that are readily available and seeing wide usage.
-
- Addresses peak compression in GELC and offers the reader a plate height equation to work with that incorporates its effects.
Frequently asked questions
How do I cancel my subscription?
Can/how do I download books?
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
What is the difference between the pricing plans?
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
What is Perlego?
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Do you support text-to-speech?
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Is Advances in Chromatography an online PDF/ePUB?
Yes, you can access Advances in Chromatography by Nelu Grinberg, Peter W. Carr, Nelu Grinberg, Peter W. Carr in PDF and/or ePUB format, as well as other popular books in Naturwissenschaften & Analytische Chemie. We have over one million books available in our catalogue for you to explore.
Information
1 Recent Advances in Supramolecular Affinity SeparationsAffinity Chromatography and Related Methods
Ashley G. Woolfork, Sazia Iftekhar, Susan Ovbude, Kyungah Suh, Sadia Sharmeen, Isaac Kyei, Jacob Jones, and David S. Hage
DOI: 10.1201/9781003223405-1
Contents
1.1 Introduction
1.2 Supports for Affinity Chromatography
1.2.1 Natural Supports and Related Materials
1.2.2 Inorganic Supports
1.2.3 Synthetic Supports
1.2.4 Magnetic Beads and Particles
1.2.5 Smart Materials
1.2.6 Nanomaterials
1.2.7 Support Formats
1.3 Immobilization Methods
1.3.1 Non-Covalent Immobilization
1.3.2 Covalent Immobilization
1.4 Binding Agents Used in Affinity Chromatography
1.4.1 Biological Agents as Affinity Ligands
1.4.1.1 Immunoaffinity Chromatography
1.4.1.2 Immunoglobulin-Binding Proteins
1.4.1.3 Lectins
1.4.1.4 Enzymes
1.4.1.5 Serum Proteins
1.4.1.6 Biotin, Avidin, and Streptavidin
1.4.1.7 Carbohydrates
1.4.1.8 Lipids
1.4.1.9 Nucleic Acids
1.4.2 Non-Biological Agents as Affinity Ligands
1.4.2.1 Boronates and Related Mixed Ligands
1.4.2.2 Dye-Ligands
1.4.2.3 Immobilized Metal-Ion Chelates
1.4.2.4 Molecularly Imprinted Polymers
1.5 Applications of Affinity Chromatography
1.5.1 Preparative Applications
1.5.1.1 Enzyme and Protein Purification
1.5.1.2 Purification of Viral Particles, Cells, and Related Targets
1.5.2 Analytical Applications
1.5.3 Chiral Separations
1.5.4 Biointeraction Studies
1.5.4.1 Zonal Elution Methods
1.5.4.2 Frontal Analysis Methods
1.5.4.3 Methods for Kinetic Studies
1.6 Conclusions
Acknowledgments
References
1.1 Introduction
Affinity chromatography is a specific type of liquid chromatography in which the separation mechanism is based on an interaction between an immobilized and biologically related binding agent with an applied analyte [1–3]. This method is closely tied to supramolecular interactions in that it makes use of the selective and reversible interactions that occur in many complexes that are formed in biological systems, or mimics of such systems. Examples of biological supramolecular interactions are those that take place between antibodies with their antigens, enzymes with their substrates or inhibitors, and hormones with their receptors [1–8]. Advantages of using these interactions in affinity chromatography include the high selectivity, strong binding, and good resolution that are made possible in this method for specific target compounds [5–8]. The stationary phase in affinity chromatography, which makes up the immobilized component of the supramolecular complex, is often called the “affinity ligand”. The support that contains this stationary phase is packed or placed into a column and used to selectively retain the complementary target to the affinity ligand, where this target represents the second part of the supramolecular complex [5–7].
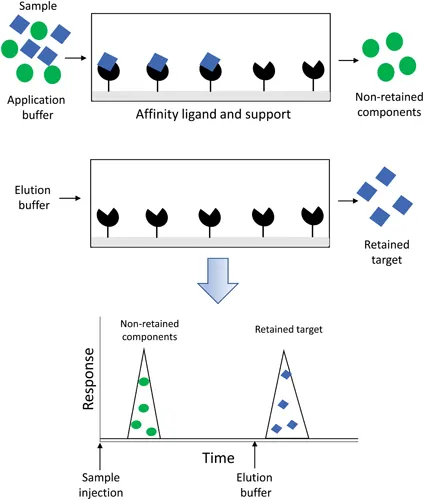
The most common format in which immobilized ligands and columns are used in affinity chromatography is the on/off elution mode, as shown in Figure 1.1 [5, 9]. In this format and in the presence of an application buffer, a sample mixture containing the target analyte is applied to the column. Non-retained components pass through the column and are quickly eluted, while the target interacts with the immobilized affinity ligand. To obtain and release the captured agent, a separate elution buffer is typically used in a situation where the analyte and binding agent have strong interactions, such as occurs for systems with association equilibrium constants of 106 M−1 or higher [1, 7–9]. To weaken these interactions, the elution buffer may have a change from the application buffer in its pH, polarity, or ionic strength; this approach is known as “non-specific elution”. Alternatively, the elution buffer may contain a competing agent to promote analyte elution by mass action, giving a method referred to as “biospecific elution” [5, 9]. After the analyte has been released, the column may then be regenerated by reapplying the original application buffer or some similar solution [5, 9]. The system is then ready for the next sample to be processed. In weak affinity chromatography (WAC), the same mobile phase is used for both sample application and elution under isocratic conditions, as well as for column regeneration. This situation is possible when the supramolecular interactions that are involved in retention have an association equilibrium constant that is less than approximately 105 or 106 M−1 [1, 7, 9–11].
Affinity chromatography has been popular for decades as a tool for the selective purification of biological molecules [1–7]. In addition, this technique has been used as a method for sample preparation during chemical or biochemical analysis and as a tool for the isolation, measurement, or characterization of targets in biological, clinical, and environmental samples [1–6,12–16]. This review will discuss recent developments in affinity chromatography and related supramolecular separation methods. Topics that will be discussed will include advances with regard to the types of supports, biological binding agents, and immobilization methods that are employed in this method. New developments in the applications of affinity chromatography will also be examined.
1.2 Supports for Affinity Chromatography
Many materials and matrices have been utilized as supports in affinity chromatography. These supports can be divided into three main categories (see Table 1.1) [1–3,17, 18]. The first category is natural supports, such as agarose, dextran, and cellulose. The second group is inorganic matrices, such as silica or glass. The third group is made up of synthetic materials that can be used as chromatographic supports, such as polystyrene or polyacrylamide [1, 17, 18].
| General Type of Support | Examples of Supports |
|---|---|
Natural | Agarose, cellulose, dextran, agarose-chitosan composites |
Inorganic | Silica, aluminum oxide, titania |
Synthetic | Polystyrenes, polyacrylamides, polysulfones, polyamides, cryogels |
Miscellaneous | Magnetic beads and particles (e.g., based on iron oxides) |
Smart materials (e.g., thermoresponsive polymers) | |
Nanomaterials (e.g., multi-walled and single-walled carbon nanotubes) |
1.2.1 Natural Supports and Related Materials
Agarose is the most popular support material used in affinity chromatography for both the large- and small-scale purification of targets [3, 19–23]. This was also the material used in the first modern separations that were described for affinity chromatography in 1968 [3, 8, 19–21]. Agarose has a large pore size, which makes it suitable for many biomedical separations or the immobilization of large biomolecules. The low cost, low non-specific binding of this material for many biological agents, and good stability of agarose over a broad pH range also make agarose appealing as a support for man...