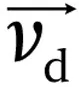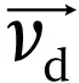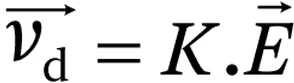![]()
CHAPTER 1
Ion Mobility–Mass Spectrometry: an Overview
Valérie Gabelica*a
a Univ. Bordeaux, CNRS, INSERM, ARNA, UMR 5320, U1212, IECB, F-33600 Bordeaux, France,
*E-mail:
[email protected] Ion mobility spectrometry is increasingly often coupled to mass spectrometry measurements, either for separation purposes or to assist compound identification. This chapter introduces basic definitions and concepts underlying ion mobility spectrometry. The definition of “collision cross-sections” as used in ion mobility spectrometry is also discussed, with a cautious note that the IUPAC definition is not entirely suited to describe the physical quantity on which ion mobility depends. Finally, the types of ion mobility analyzers most commonly encountered in contemporary commercial ion mobility-mass spectrometers are introduced and compared.
1.1 What is Ion Mobility Spectrometry?
1.1.1 Spectrometry
A spectrometric technique physically separates compounds in a so-called spectrometer. A spectroscopic technique, in contrast, analyses the interaction between matter and electromagnetic radiation (UV, visible, infrared light, etc.).
The most widespread spectrometric technique is mass spectrometry, which physically separates compounds according to their mass-to-charge ratio. In practice, mass spectrometry separates ions, not neutral compounds, because the separation is achieved by the movement of ions in an electric or magnetic field. To ensure that the ion movement is defined only by the electric or magnetic field, as desired in most mass analysis approaches, mass spectrometers operate at low pressure so that collisions do not interfere with the movement of the ions during mass analysis.
1.1.2 Ion Mobility
Imagine you want to move ions using an electric field (E⃑). The electric force applied to the ions is F⃑ = qE⃑. The higher the charge q, the higher the force F⃑. Because of the electric field, the ions will accelerate, according to the law F⃑ = ma⃑, where a⃑ is the acceleration.
Now let us imagine that the ions are accelerated in a medium filled with gas, at a high enough gas pressure that there are many collisions to compensate for the acceleration. Because of the collisions, the ions will slow down. The collisions are responsible for a friction force, acting in the opposite direction to the applied electric force. So, when ions are subjected to an electric field in a region of relatively high pressure, they will constantly be accelerated, decelerated, accelerated, decelerated, and so on (Figure 1.1).
Figure 1.1The instant velocity of an ion is constantly changing, and the average drift velocity
depends on the balance between accelerations by the electric field
E⃑ and decelerations by collisions.
If the collisions are frequent and numerous enough, the electric force and friction force balance each other and a
stationary state is reached. As the two forces cancel each other out, there is no net acceleration, and the average speed will appear constant. This is called the
drift velocity (
).
The ion’s mobility (
K) is the proportionality constant between the drift velocity and the electric field:
In summary, ion mobility spectrometry consists of separating ions in an electric field in the presence of a collision gas. The separation will be based on the value of K, the ion’s mobility. This chapter will cover the very basics of ion mobility spectrometry. For a thorough coverage of ion mobility theory, the reader can refer to a recent book by Larry A. Viehland.1
1.2 What is Ion Mobility Spectrometry Used For?
The mobility, K, of an ion depends on its charge (q = ze, where z is the net charge and e the charge of an electron), and its friction in the gas. We are interested in measuring this friction. Indeed, even if friction is partly related to mass (ions of higher mass are usually larger as well), other parameters come into play, for example the arrangement of atoms in space (the three-dimensional structure) of the ion. At equal mass and charge, if an ion has a more expanded structure, the friction will be greater, thus the mobility will be smaller, and the drift velocity will be lower. If the ion has a more compact structure, its mobility will be larger, resulting in a higher drift velocity.
This is the “parachute” effect. If you jump out of an airplane, you are subjected to the force of gravity. But you can slow down your fall by deploying your parachute. Your mass does not change, the force of gravity does not change, but your “conformation” changes and slows you down. The larger your parachute, the slower your fall (Figure 1.2).
Figure 1.2An ion mobility analogy: the larger your parachute, the slower your fall.
Ion mobility spectrometry separates ions according to their three-dimensional structure (shape, i.e. nuclei positions but also, as we will see below, electronic structure), and thus provides complementary information to mass spectrometry. Ion mobility spectrometry is particularly useful for separating isomeric compounds (which have the same atoms but different three-dimensional arrangements), or isobaric compounds (which incidentally have the same mass). Since both spectrometries are performed on ions in the gas phase, they are frequently coupled into a single instrument: an ion mobility–mass spectrometer (IM-MS). Consequently, ion mobility spectrometry can be used for many different applications, as detailed below.
1.2.1 An Additional Method of Separation Coupled to Mass Spectrometry
The ion mobility separation typically takes place on the millisecond time scale,
i.e. orders of magnitude faster than chromatography, and thus the methods can be used orthogonally. The IM-MS combination is particularly useful:
- To resolve conformational isomers. Figure 1.3 shows an example with the separation of two conformations for the dinucleotide dCG (deoxycytosine–deoxyguanine).2 Here the mobility separation was carried out in a temperature-controlled drift tube. Folded conformations travel faster (arrive earlier) than open conformations, but it is interesting to note that the two peaks are separated only at low drift tube temperature. This illustrates that one condition for separating conformers by ion mobility spec...