
eBook - ePub
Solid-Liquid Thermal Energy Storage
Modeling and Applications
Moghtada Mobedi, Kamel Hooman, Wen-Quan Tao, Moghtada Mobedi, Kamel Hooman, Wen-Quan Tao
This is a test
Share book
- 346 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Solid-Liquid Thermal Energy Storage
Modeling and Applications
Moghtada Mobedi, Kamel Hooman, Wen-Quan Tao, Moghtada Mobedi, Kamel Hooman, Wen-Quan Tao
Book details
Book preview
Table of contents
Citations
About This Book
Solid – Liquid Thermal Energy Storage: Modeling and Applications provides a comprehensive overview of solid–liquid phase change thermal storage. Chapters are written by specialists from both academia and industry. Using recent studies on the improvement, modeling, and new applications of these systems, the book discusses innovative solutions for any potential drawbacks.
This book:
-
- Discusses experimental studies in the field of solid–liquid phase change thermal storage
-
- Reviews recent research on phase change materials
-
- Covers various innovative applications of phase change materials (PCM) on the use of sustainable and renewable energy sources
-
- Presents recent developments on the theoretical modeling of these systems
-
- Explains advanced methods for enhancement of heat transfer in PCM
This book is a reference for engineers and industry professionals involved in the use of renewable energy systems, energy storage, heating systems for buildings, sustainability design, etc. It can also benefit graduate students taking courses in heat transfer, energy engineering, advanced materials, and heating systems.
Frequently asked questions
How do I cancel my subscription?
Can/how do I download books?
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
What is the difference between the pricing plans?
Both plans give you full access to the library and all of Perlego’s features. The only differences are the price and subscription period: With the annual plan you’ll save around 30% compared to 12 months on the monthly plan.
What is Perlego?
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Do you support text-to-speech?
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Is Solid-Liquid Thermal Energy Storage an online PDF/ePUB?
Yes, you can access Solid-Liquid Thermal Energy Storage by Moghtada Mobedi, Kamel Hooman, Wen-Quan Tao, Moghtada Mobedi, Kamel Hooman, Wen-Quan Tao in PDF and/or ePUB format, as well as other popular books in Naturwissenschaften & Energie. We have over one million books available in our catalogue for you to explore.
Information
1 An Introduction to Solid–Liquid Thermal Energy Storage Systems
Moghtada Mobedi
Shizuoka University
Kamel Hooman
The University of Queensland
Wen-Quan Tao
Xi’an Jiaotong University
DOI: 10.1201/9781003213260-1
Contents
- 1.1 Introduction
- 1.2 Classification of Thermal Energy Storage
- 1.3 Difficulties Associated with Solid–Liquid Thermal Storage
- 1.3.1 Challenges with PCM
- 1.3.2 System Design Challenges
- 1.4 Solid–Liquid Thermal Energy Storage Applications
- 1.5 Conclusion
- Acknowledgment
- References
1.1 Introduction
With global population growth and our modern lifestyle, our energy demand keeps increasing. Our ever-increasing demand for energy should be met by renewable energy for a sustainable future. Both sustainability and environmental concern contributed to the growth of renewable energy at the international level, while most of the countries set national goals and made significant progress. In Australia, for example, 27.7% of the generated electricity was from renewable sources in 2020 with 50% annual increase in solar generation [1]. Tasmania, the Australian island state with just over 10 TWh annual energy demand, is currently running on 100% renewable energy. Heavily relying on wind, renewables contribute to 90% of the electricity supply in Scotland. Over the first quarter of 2020, clean energy sources contributed to almost half of national electricity generation in the United Kingdom. Similarly, over the first three quarters of the same year, renewables contributed to almost half of Germany’s electricity consumption. Currently, across all the key sectors, i.e. power, heating, industry and transport, demand for renewables is on the rise. The power sector, in particular, is in the lead with its demand for renewables increasing by 8% to reach 8300 TWh; the highest annual growth which was recorded in 2020, according to the IEA (International Energy Agency) [2]. Two-thirds of this amazing renewables growth is attributed to wind and solar PV (photovoltaic); expectedly both sectors are expected to expand in 2021. For instance, over 900 TWh is expected to be generated from these two sources in China this year. These numbers are 589 and 550 TWh for the European Union and the United States, respectively. On global scale, solar PV and wind grew by 12% and 23%, respectively, in 2020. As anticipated, the levelized cost of electricity keeps dropping thanks to technology and market development. However, the capacity factor, for either of solar PV or wind, is too low to allow for baseload energy production with no storage. Battery storage remains essential yet prohibitively expensive. Hence, alternative forms of energy storage including thermal energy storage are called for.
Ultimately, with our move toward energy generation from renewable sources, energy storage remains the key issue. This is mainly because most of the renewable energy sources are intermittent in nature. It can be claimed that a fully renewable energy future is impossible without inexpensive and reliable storage of energy. While chemical, electrical, mechanical and potential energy storage options have been investigated before, the focus of this book is on thermal energy storage in phase change materials (PCMs). Within thermal energy storage techniques, we limit the scope of this book to those relying on phase change. In particular, systems with phase transition from a solid to a liquid state are to be investigated. Applications for such system encompass a wide range including food safety and transport, building energy management, electronic cooling, waste heat recovery, health and well-being, refrigeration, solar thermal power generation, automobile industry, as well as thermal management of satellites. There are certain challenges to be addressed before these PCM-based technologies can be commercialized and we will cover some of those later on. On top of practical questions, there are fundamental research questions to be answered. Some of these questions center on the tools we use to analyze the thermo-chemo-mechanical behavior of thermal storage systems. This book will offer a combination of theoretical, numerical and experimental techniques to address these questions in forthcoming chapters. Moreover, while development of a technology is important, its deployment and market success will depend on a number of parameters among which cost can be investigated and minimized as will be shown in this book.
1.2 Classification of Thermal Energy Storage
Thermal energy storage (TES) technologies may be classified according to following four aspects: working temperature, duration of storage, circulation of heat transfer medium and energy storage mechanism [3, 4, 5] (Figure 1.1).
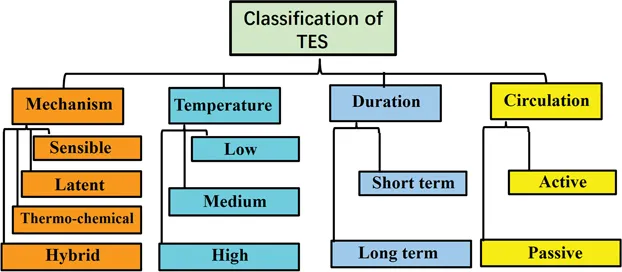
- Temperature: When the stored energy temperature is below 100°C, the system may be referred to as a low temperature energy storage one. When the stored energy temperatures are in 100°C–200°C or beyond 250°C, the terms medium and high temperature energy storage are used, respectively.
- Duration: If the energy storage system is charged or discharged daily or weekly, it is called short-term storage. While in a long-term storage system energy is stored for months or seasons [6].
- Circulation: Since the TES system usually requires heat transfer fluid (HTF) during the charging and discharging process, an obvious question would be about the driving force for moving HTF in the loop. If the HTF is circulated by buoyancy effects without external force (such as a fan or a pump), the storage system is called passive, while in an active system, the HTF is driven by an external force.
- Mechanism: The TES mechanism is, arguably, the most important aspect of the classification [7, 8, 9, 10]. Here, we use the following four indicators for our classifications, and the technology used for energy storage can be further subdivided into four different types (Figure 1.2).
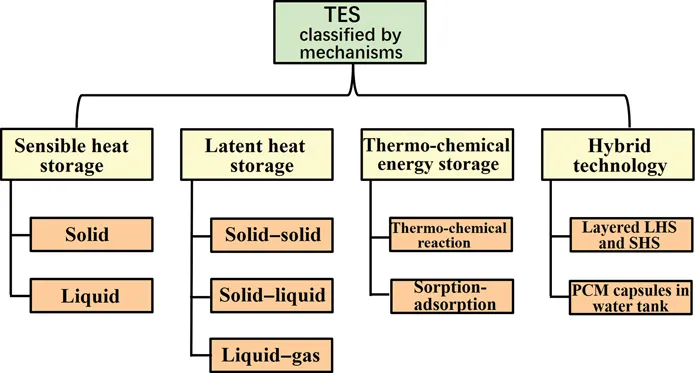
FIGURE 1.2 Further classification of TES by mechanism.
In the sensible heat storage (SHS), heat may be stored in solids (rocks, concrete, bricks, sand, or metals). Heat can also be stored in liquids: most often liquids water, molten salts and mineral oils. In the latent heat storage (LHS), energy is stored, in PCMs, when material changes its state from solid to solid [11], solid to liquid or from liquid to gas while the solid-liquid phase change is the most widely adopted technology, hence the exclusive focus of this book. The frequently used PCMs are organic, inorganic and eutectic materials, as well as metallic liquids which have been studied at laboratory scale [12] (Figure 1.3).
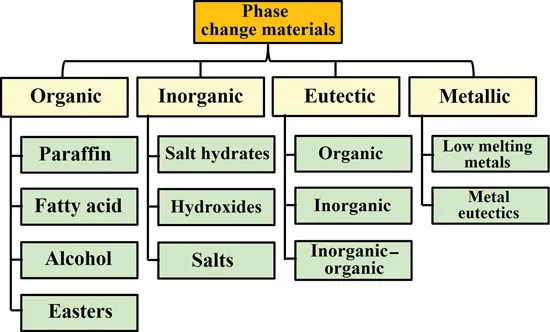
Relying on high latent heat for PCMs is essential. Ideally, a low mass of the PCM should hold as much as possible of latent heat of fusion. The phase change process initiates at a given melting point, and the released heat must be conducted through the solid and liquid phases. In view of the above, these three, melting temperature, latent heat and thermal conductivity, can be considered as the major thermophysical properties of PCMs [13]. Thermophysical properties of some PCMs are presented in Table 1.1 [12] and, in the interest of brevity, only the three major ones are presented here.
| Phase Change Materialsm | Classification | Temperature, °C | Latent Heat of Fusion, kJ/kg | Conductivity, W/(m·K) |
|---|---|---|---|---|
| Paraffin wax (C13–C18) | Organic | 32 | 251 | 0.214 |
| Polyglycol E600 | Organic | 22 | 127.2 | 0.189 |
| Vinyl stearate | Organic | 29 | 122 | 0.25 |
| Butyl stearate | Organic | 19 | 140 | 0.21 |
| 1-Dodecanol | Organic | 26 | 200 | 0.169 |
| Octadecane | Organic | 28 | 243.5 | 0.148 |
| Palmitic acid | Organic | 57.8 | 185.4 | 0.162 |
| Capric acid | Organic | 32 | 152.7 | 0.153 |
| Caprylic acid | Organic | 16 | 148 | 0.149 |
| Propyl palmitate | Organic | 10 | 186 | - |
| KNO3/NaNO3 | Inorganic | 220 | 100.7 | 0.56 |
| CaCl2·6H2O | Inorganic hydrates | 29 | 187 | 0.53 |
| LiNO3/KNO3/NaNO3 | Inorganic eutectic mixture | 121 | 310 | 0.52 |
| (a) KNO3/LiNO3 | Inorganic eutectic mixture | 124 | 155 | 0.58 |
| (b) KNO3/NaNO3/LiNO3 | Inorganic eutectic mixture | 130 | 276 | 0.56 |
| LiNO3/CaNO3 with KNO3/NaNO3 (30%/70%) | Inorganic noneutectic mixture | 260 | 305 | 0.54 |
| Capric acid and lauric acid | Organic eutectic mixture | 19.67 | 126 | 0.21 |
| Polymethyl methacrylate/capric-stearic acid mixture | Organic eutectic mixture | 21.37 | 116.25 | 0.15 |
Thermochemical storage relies on thermochemical reaction storage and sorption storage. In the thermochemical energy storage system, the thermal energy is first supplied to the thermochemical material, say C, to dissociate it into two products, say A and B. This charging process is an endothermic reaction. Then the two compounds A and B are stored separately. During the discharge process, the two compounds are reunited again and the stored energy is released thanks to an exothermic reaction. The studied thermochemical materials include MgSO4, FeCO3, and CaCO3.
Sorption can be thought of as a process of capturing a gas or a vapor (called sorbate) by a substance (solid or liqui...