![]()
CHAPTER 1
Unique Nature of Phosphate and Borate Bioactive Glasses
TOSHIHIRO KASUGA*a
a Nagoya Institute of Technology, Showa-ku, Nagoya 466-8555, Japan,
*E-mail:
[email protected] In recent years, as new fields in biomedical applications have emerged, considerable attention has been devoted to glass materials for their ability to promote bone formation and their application for the treatment and repair of soft tissue. So far, silicate-based glasses have been widely applied as biomedical glasses. In some cases, however, silicate-based glasses are not suitable for all applications. Phosphate-based and borate-based glasses are proposed as attractive alternatives. They are prospective biomaterials, because their structures are controllable and their characteristic ion-releasing behaviour can be tuned.
1.1 Phosphate Glasses for Biomedical Applications
Phosphate glasses have structural features that are significantly different from those of silicate glasses. Phosphate glasses typically consist of a layered or chain structure in which PO4 units are connected and, with a few exceptions, do not form a three-dimensional PO4 network. This is because P is pentavalent and can form a 4-coordinated structure with oxygen by forming a dπ–pπ-type bond with one of the four coordinating oxygens. Therefore, non-bridging oxygens (NBOs) are naturally present in phosphate glasses, eliminating the requirement of introducing modifier components such as alkali oxides. The introduction of modifiers leads to the formation of various phosphate groups such as orthophosphate units, Q0, pyrophosphate units, Q1, metaphosphate (intermediate) units, Q2, and branching units, Q3. The difference in π-bonding and σ-bonding properties of each group influences the chemical properties of the phosphate glasses. Q3 is chemically unstable because the double bond moiety is localised to a single P–O bond, and it easily reacts with water to convert to Q2 delocalised to two P–O bonds. This is called the “anti-branching rule”.1 This delocalisation of the double bond can be verified through X-ray photoelectron spectroscopy (XPS) by using the O 1s spectra2 and via electron spin resonance (ESR) spectroscopy.3 In M2O–P2O5 (M = alkali metal cation) glasses, upon increasing the M2O content to a composition at which Q3 groups disappear, such glasses become more chemically stable, in contrast to conventional silicate glasses. When glasses with an ultraphosphate composition are melted, moisture in the raw materials and in the atmosphere is taken up. The (M2O + H2O)/P2O5 ratio tends to approach unity, following the “anti-branching rule”, to eliminate Q3 branching groups. As a result, phosphate glasses include a larger number of OH groups than silicate or borate glasses do.4
Because phosphate glasses have lower electron densities on oxygen than silicate glasses and are more acidic, they can be used as good solvents for basic oxides and serve as hosts with weaker ligand fields. Moreover, it is considered that these structural features also contribute to the good glassification ability, even when the P2O5 amount is 40% or less, and to the dissolution of oxide compounds, such as Ag2O, at high concentrations. The chemical durability of phosphate glasses can be considerably improved by incorporating B2O3 and/or Al2O3 to improve the symmetry around tetrahedral PO4, and various practical glasses such as ultraviolet transparent glasses or sulfur-resistant glasses have been developed. The detailed structure and properties of phosphate glasses for biological use are described by Delia Brauer in Chapter 2.
The structure of phosphate glasses has been described in many reviews,5,8 including Van Wazer9 and Abe10 et al. Therefore, in this chapter, the author will introduce recently reported phosphate glasses with interesting specific structures and properties.
1.1.1 Invert Glasses
Unlike silicate and borate glasses, phosphate glasses can be vitrified even when their composition comprises a small amount of so-called “network former” (NWF) ions. When appropriate compositions are chosen, phosphate glasses consisting of Q0 and/or Q1 units can be obtained without polymerisation of PO4 tetrahedra.8,10 Glass “networks” are formed by PO4 tetrahedra being ionically connected via metal cations. In other words, it is difficult to form a glassy state only with the Q0 and/or Q1 units; however, phosphate glasses can be vitrified by incorporating other components that play the role of a network former. These glasses are examples of systems in which Zachariasen's “random network model” cannot be applied, and are classified as “invert glasses”, as proposed by Trap and Stevels.11 Here, independent anionic groups are linked by cations that were originally network modifier (NWM) components; the anionic groups act as NWM-like groups and the cations are functionally considered to be NWF. For biomedical application, 60CaO–30P2O5–10TiO2 (in mol%) glass12 has been reported as one of the ideal glasses. This glass exhibits in vitro bioactivity (apatite-forming ability in a simulated body fluid). This glass and its glass-ceramic with a modified composition have been reported to adhere directly to bone in animal experiments.13
Maeda et al. 14 have clarified the effect of TiO2 in a calcium phosphate invert glass on the local structure by using spectroscopic methods and simulations based on molecular dynamics (MD). From 31P magic angle spinning nuclear magnetic resonance (MAS-NMR) spectra, Q1 and Q2 peaks were observed in the 60CaO–40P2O5 glass, whereas Q0 and Q1 peaks were observed in the 60CaO–30P2O5–10TiO2 glass. Malavasi et al. 15 reported the network connectivities for 60CaO–40P2O5 and 60CaO–30P2O5–10TiO2 glasses to be 1.42 and 0.77, respectively. The shortening of the phosphate chain structure by incorporating TiO2 was considered to cause a decrease in network connectivity. In the Raman spectrum of the 60CaO–30P2O5–10TiO2 glass, peaks attributed to the TiO4 and TiO6 units were observed.
In the XPS O1s spectrum of the 60CaO–40P2O5 glass, peaks attributed to P–O–P and P–O–Ca bonds were observed, while in that of the 60CaO–30P2O5–10TiO2 glass, the P–O–Ti peak was observed in addition to the P–O–P and P–O–Ca peaks. These results suggest that in the 60CaO–30P2O5–10TiO2 glass, 4- and 6-fold coordinated titanium ions exist and form P–O–Ti bonds with the phosphate species.
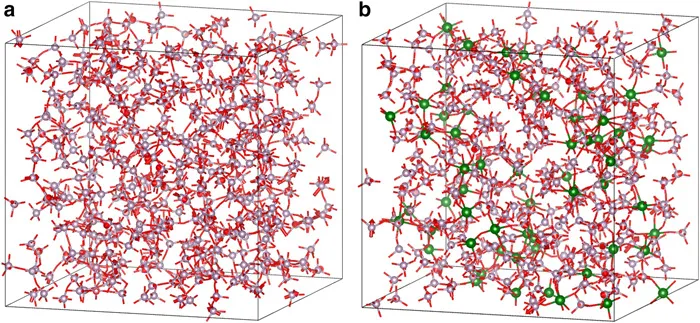
Figure 1.1 shows the structural model of the glasses obtained using the MD method. In this figure, the structures wherein P, Ti and O are contributing to the glass network structure are extracted. The network connectivities for 60CaO–40P2O5 and 60CaO–30P2O5–10TiO2 glasses were 1.53 and 0.67, respectively, which are close to the spectroscopy results. Because the formation of the P–O–Ti bond is considered in the XPS measurement, TiO2 also acts as an NWF. Assuming that P2O5 and TiO2 work to form the network structure of the 60CaO–30P2O5–10TiO2 glass, its connectivity is 1.81, which is higher than that for the 60CaO–40P2O5 glass.
Figure 1.1 Snapshots of (a) 60CaO–40P2O5 and (b) 60CaO–30P2O5–10TiO2 glass models simulated using 2000 atoms. Ca atoms are not shown for easy viewing. Colour legend: P (purple), Ti (green), and O (red bar).
Water wettability, which is a functional indicator for biomaterials, of 60CaO–40P2O5 and 60CaO–30P2O5–10TiO2 glasses was evaluated to be 75.0° ± 4.4° and 54.0° ± 4.9°, respectively. The incorporation of TiO2 increases the network connectivity of the glass, resulting in an increase in the bond strength of the entire glass. This increases the surface tension, which contributes to the improvement of hydrophilicity. The elucidation of the local structure of phosphate glasses has allowed its correlation with their biological functions.
The development of this invert glass has led to the investigation of new biomedical glasses, which are described in detail by Sungho Lee in Chapter 5.
1.1.2 Phosphate Glasses Containing 6-fold Coordinated Silicon Structure
It has been reported that the incorporation of TiO2 and CaO improves the chemical durability of phosphate glasses, thus widening their scope for biomedical application.16,17 Recently, silicate ions have been recogni...