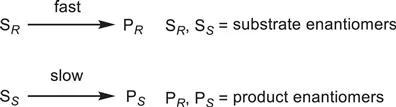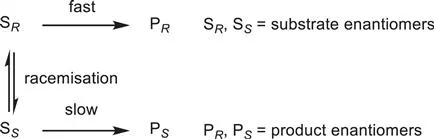This book collects, for the first time, all of the developments focussing on organocatalysed dynamic kinetic resolutions, demonstrating the blooming of this special field that joins two powerful concepts, namely organocatalysis honoured by the 2021 Nobel Prize in Chemistry and dynamic kinetic resolution. The growing economic importance of chiral molecules, especially in medicine, has spurred major research efforts towards the selective preparation of enantiopure products. The resolution of racemates still constitutes the most employed methodology to prepare chiral products in industry in spite of the huge expansion of asymmetric synthesis and especially enantioselective catalysis. However, the principal drawback of a simple kinetic resolution is related to the limitation of the yield to 50%. Attempts to overcome this limitation have been undertaken, resulting in the discovery of dynamic kinetic resolution, which allows 100% yield through the combination of a resolution step of a kinetic resolution with an in situ racemisation of the chirally-labile substrate performed in the presence of a catalyst. Along with enzymes and metals, this catalyst can be a non-toxic, inexpensive, robust and often readily available chiral organocatalyst. The first examples of organocatalysed dynamic kinetic resolutions have been developed in the last two decades. This book presents in eight chapters dynamic kinetic resolutions organocatalysed by cinchona alkaloids, proline and other amino acid-derivatives, phosphoric acids, N-heterocyclic carbenes, thioureas, pyridine-based Lewis bases, tetramisoles, and miscellaneous organocatalysts.
The preparation of chiral compounds constitutes one of the most important and fascinating fields of contemporary synthetic organic chemistry, related to the fact that most natural products are chiral and their physiological or pharmacological properties depend upon their recognition by chiral receptors, which will interact only with molecules of the proper absolute configuration.1 The use of drugs in enantiopure form is today a standard requirement for virtually every new chemical entity, and the development of new synthetic methods to obtain chiral compounds has become a key goal for pharmaceutical companies. The growing economic importance of chiral molecules in medicine, and also in materials, has spurred major research efforts towards the selective preparation of chiral compounds. Over the last three decades, an explosive growth of research in the area of asymmetric synthesis has occurred. Asymmetric synthesis constitutes one of the main strategies to gain access to enantioenriched compounds, involving the use of either chiral auxiliaries or catalysts derived preferentially from cheap chiral pool sources. In particular, asymmetric catalysis of organic reactions to provide enantiomerically enriched products is of central importance to modern synthetic and pharmaceutical chemistry. Moreover, the resolution of racemates still constitutes the most employed methodology to prepare chiral products in industry, in spite of the huge expansion of asymmetric synthesis and especially enantioselective catalysis. In a simple kinetic resolution, one enantiomer (SR) of a racemic mixture is more rapidly transformed into the corresponding chiral product (PR), while the other (SS) is recovered unchanged, as illustrated in Scheme 1.1.2
Scheme 1.1 Simple kinetic resolution.
The principal drawback of this methodology is related to the limitation of the yield to 50%. Attempts to overcome this limitation have been undertaken, resulting in the discovery of dynamic kinetic resolution. Indeed, this methodology allows a quantitative yield of one of the enantiomers to be achieved. Actually, dynamic kinetic resolution combines the resolution step of a kinetic resolution with an in situ equilibration or racemisation of the chirally-labile substrate (Scheme 1.2). The enantiomers of a racemic substrate are induced to equilibrate at a rate that is faster than that of the slow-reacting enantiomer in reaction with the chiral reagent (Curtin–Hammett kinetics). If the enantioselectivity is sufficient, then isolation of a highly enriched non-racemic product is possible with a theoretical yield of 100% based on the racemic substrate. Special requirements have to be fulfilled in order to gain the complete set of advantages of dynamic kinetic resolution, such as the irreversibility of the resolution step, and the fact that no product racemisation should occur under the reaction conditions. In order to obtain products with high enantiopurity, the selectivity (kA/kB) of the resolution step should be at least 20. Furthermore, the rate constant for the racemisation process (kinv) should be faster than the rate constant of the resolution step (kA), otherwise a very high selectivity has to be ensured.
Scheme 1.2 Dynamic kinetic resolution.
Under these conditions, the two starting enantiomers of the racemic mixture can be converted into a single enantiopure product in 100% theoretical yield. The required racemisation of the substrate can be performed either chemically, biocatalytically or even spontaneously. However, the conditions must be chosen to avoid the racemisation of the formed chiral product. The utility of dynamic kinetic resolution is not limited to a selective synthesis of an enantiomer; when the reaction occurs along with the creation of a novel stereogenic center, the stereoselective synthesis of a diastereoisomer is also possible. This powerful concept has been applied to both enzymatic3 and non-enzymatic reactions.4 Along with enzymes3 and metal catalysts,5 organocatalysts present considerable advantages in addition to being environmentally compatible, since they are non-toxic, inexpensive, robust and often readily available.6 In particular, their use in the synthesis of drugs is highly appreciated thanks to the exclusion of any trace of hazardous metals in the final products. This type of green catalyst has been applied in the last two decades to describe the first examples of organocatalysed dynamic kinetic resolutions, allowing a considerable extension of the synthetic scope of the dynamic kinetic resolution methodology. While the end of the last century has been dominated by the use of metal catalysis,7 a drastic change in perception occurred in the 1970s when the pioneering work independently reported almost simultaneously in 1971 by Hajos6c,8 and Wiechert6b,9 demonstrated for the first time that simple chiral small organic molecules, such as l-proline, are able to promote organic transformations in a highly enantioselective manner. These pioneering works opened the door to modern, clean, environmentally compatible and highly efficient asymmetric organocatalysis, which has become an outstanding, powerful, fascinating and highly efficient novel tool in green organic chemistry. Indeed, metal-free organic catalysts are generally non-toxic, inexpensive, readily available and stable in comparison with metal catalysts, which are often toxic and expensive. These properties allow most reactions to be performed in a wet solvent or air, increasing the operational simplicity of these green processes. Unfortunately, this remarkable discovery did not receive the attention that it deserved at the time and remained curiously undeveloped for almost 30 years until 2000, when List6e and MacMillan,6d honoured with the 2021 Nobel Prize in Chemistry, relaunched this revolutionary field. This renaissance has prompted the organic chemistry community to participate in the explosion of green organocatalysis, which has definitively matured to become a recognised third methodology of potentially equal status to organometallic and enzymatic catalysis, allowing the clean synthesis of many drugs and natural products without metal contamination. Asymmetric organocatalysis can follow different modes of activation, which can be classified according to the covalent or noncovalent character of the substrate–organocatalyst interaction and to the chemical nature of the catalyst (Lewis base, Lewis acid, Brønsted base, Brønsted acid). Furthermore, a wide range of organocatalysts ca...