![]()
CHAPTER 1
Dissecting Daylight: How We See Color†
The history and usage of pigments are intimately related to the development of technology, physics and chemistry. This chapter introduces some of these technical details, including an introduction to the nature of light, its characteristics and relationship to the three-color physiology of the human eye. Color mixing, how light interacts with matter, and how this relates to our primary interest, pigments, make up the remainder of the chapter. Some technical details are, necessarily, presented, but skipping them will not hinder you from plunging into the rest of the pigment story.
Colour is the place where our brain and the universe meet. Paul Cézanne.1
1.1 Introduction
The history and usage of pigments are intimately related to the development of technology, physics and chemistry. This chapter introduces some of these technical details, including the nature of light and how it interacts with the human eye and colored substances, and how pigments as colored substances fit in. You can easily skip the technical details of this chapter without detracting from the joy of the pigment journey you are about to embark upon.
1.2 E.T. Revisited?
If you looked up into Earth's big blue sky on 19 October 2017, you may have sighted a potential visitor from far beyond the solar system. The object, shaped like a cigar and dubbed 1I/2017 U1, was sighted just passing through our astronomical neighborhood. Harvard astrophysicist Avi Loeb lent credence to this possible “social call” by aliens in a blog post for Scientific American, later transformed into a book.2,3 If the inhabitants of 1I/2017 U1 had decided to stop off to say “hello,” and if their point of origin were a planet tied to the closest red giant, Gamma Crucis, they would have stumbled out of their spaceship functionally blind.
1.3 The Nature of Light
Why is this so? Why couldn't these aliens see non-red objects? We need light to see and there are different forms of light – the light coming from our sun is different from the light emitted by Gamma Crucis. What is the nature of this difference? To find out, we need to back up a bit, examine the nature of light, and then its relationship to color.
All light is accompanied by heat, so we infer that they share the same nature, called energy, i.e., anything that causes a change in motion. Energy exhibits many forms, such as heat, light, and electricity; all forms are interconvertible by interaction with matter. The ancient Greeks were the first to theorize regarding the nature of light and color. Aristotle (384–322 BCE) made the first important contribution to what is now the modern theory of selective absorption (Figure 1.4).4 Then Seneca (4 BCE – 65 CE), a Roman stoic philosopher, first noted that a prism reproduces the colors of the rainbow. Leonardo da Vinci (1452–1519) noticed that when light struck a water glass that it “spread out” as a colored image, but it was Isaac Newton (1643–1727) in 1666 who formulated modern color theory by experiment.
Newton passed a ray of sunlight through a prism and noted that it dispersed into an array of colors on the opposite wall. When he placed another prism in the path of the dispersed colors, they recombined to form sunlight again, what today we call “white light.” Newton had succeeded in dissecting sunlight into what we term the “prismatic” colors because they can be generated by a prism. He initially called them red, yellow, green, blue and violet2 in that order.5,6 Newton was astounded by this revelation, a seeming paradox that prompted controversy for the following 300 years.
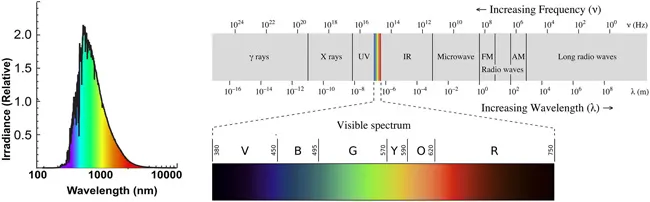
First Thomas Young (1773–1829) in 1801, and then James Clerk Maxwell (1831–1879) in 1865, theorized that light is a special form of energy that can travel through empty space at great speed3 generating electric and magnetic fields as it goes; these fields oscillate with a certain frequency (symbol ν) which defines the energy of the light beam. The distance traveled by a single oscillation is called its wavelength (symbol λ), measured in a unit called a nanometer (nm), a billionth of a meter. This is a handy measure because our sunlight has a maximum intensity in the wavelength range of about 400 to 700 nanometers. Wavelength ranges above 700 nm, which defines the limit of what we call the color red, are in the infrared (IR) region; wavelength ranges below 400 nm, which defines the limit of what we call the color violet, are in the ultraviolet (UV) region. All of these energies are present in the sun's rays, which we can now call the solar spectral irradiance curve, shown in Figure 1.1, left.7 The entire range of wavelengths, called the electromagnetic spectrum, highlighting the visible spectrum and its colors, is shown in Figure 1.1, right.
Figure 1.1Left: Solar spectral irradiance curve. The height of the curve (y-axis) shows the intensity of the radiation plotted against the wavelength in nm. Note that maximum intensity is in the green region, falling off on both sides toward the red and violet regions. Reproduced from ref. 7.4 Right: The electromagnetic spectrum showing all the regions; offset is the visible region, the only part of the spectrum that we can see. Note that the other regions of the spectrum are quite important in modern life as well. Reproduced from https://commons.wikimedia.org/w/index.php?title=File:EM_spectrumrevised.png&oldid=468982208, under the terms of the CC BY-SA 3.0 license, https://creativecommons.org/licenses/by-sa/3.0/deed.en.
1.4 How We See Color
Now, getting back to our visitors from Gamma Crucis, a star that emits a range of energy that maximizes in the IR, invisible to human eyes. But the evolutionary pattern of the light receptor cells in our visitors' eyes would have necessarily maxed out in that range (because that's where all the light is on their planet) to find food or avoid becoming someone else's dinner. Our human eyes, for the same reason – by dint of biological evolution – are sensitive to the energy range of the sun's rays of maximal intensity that actually manages to penetrate our atmosphere – namely the red, orange, yellow, green, blue and violet radiation.8,9 Hence we call this range, or spectrum of energies, the visible region (for us!). If we traveled to our Gamma Crucis visitors' planet, it would be our turn to be blind – because their solar spectral irradiance curve would maximize in the infrared region.10
All light sources are not the same, as we have seen. It depends upon the star you were born under, but also the type of lighting in your living room. Incandescent light seems “warm” to us because incandescent lamps emit a greater intensity of red light than the other colors; fluorescent lighting seems “cool” to us because it is poor in red light, but rich in yellow, green and blue light. Each type of light source has its own spectral irradiance curve that is quite different from ordinary daylight or sunlight.
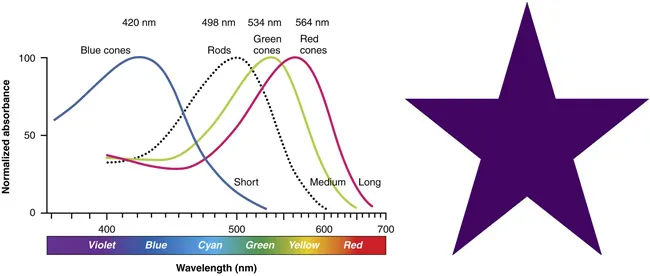
Figure 1.2 (left) illustrates how human retinal photoreceptors, consisting of rods and cones, also theorized by Young,11 respond to light stimulus.12,13 The tri-chromatic system involves retinal structures called cones that contain photopigment proteins called photopsins that are sensitive to short (blue), medium (green) and long (red) wavelengths of light respectively. Light stimulus causes these proteins to undergo a structural change that activates their ability to send an electrochemical signal to the brain. The rods, which are far more numerous and sensitive than cones, have only one photopigment, rhodopsin. Rods operate in low light to give gray images.13 Prolonged gazing at a single color can exhaust the sensitivity of the respective cones, producing an afterimage of the complementary color. If you gaze at the magenta star (a color combination of red and blue) in Figure 1.2 (right) for several minutes and then shift your gaze elsewhere, you should see a faint green afterimage – green is the complementary color of magenta.
Figure 1.2Left: Sensitivity curves for the rods and cones in the human retina. Reproduced from ref. 12, https://openstax.org/books/anatomy-and-physiology/pages/1-introduction, with permission from OpenStax under the terms of the CC BY 4.0 license, https://creativecommons.org/licenses/by/4.0/. Right: Magenta star.
1.5 Additive and Subtractive Color Mixing
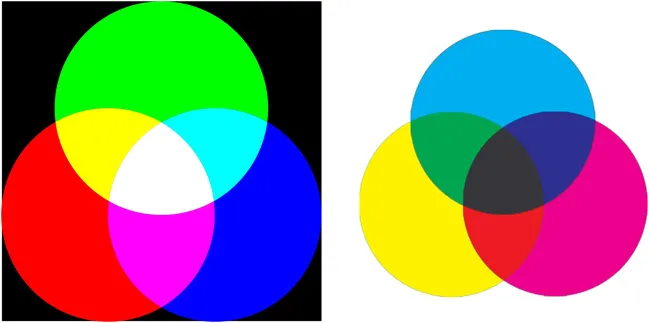
The color mixing circles shown in Figure 1.3 provide the rationale for complementary colors. Figure 1.3 (left) depicts additive color mixing, which is the mixing of lights.14 Each of the three major regions of the visible spectrum can be roughly divided into the red (600–700 nm), the green (500–600 nm) and the blue (400–500 nm) regions. If all three colored lights are added together, they will produce white light, as seen in the center of the three overlapping circles (which is why sunlight appears white: it contains all of the spectral colors). We call red, green and blue the additive primary colors. When neighboring circles overlap, they produce the complementary colors: green + blue = cyan (a turquoise color); blue + red = magenta (sometimes called purple); red + green = yellow. So the complementary colors are yellow (to blue), magenta (to green) and cyan (to red). Now can you see why staring at a magenta star overwhelmed your red and blue sensitive cones for a while, and you could only see green for a time. In Figure 1.3 (right), we see the subtractive color system. Here, the three subtractive primary colors are yellow, magenta and cyan; their respective complements, shown in the overlapping neighboring circles are, respectively, blue (magenta + cyan), green (cyan + yellow) and red (yellow + magenta). A combination of all three subtractive primaries yields black. This is the system used in four-color printing.15 Check the colors of your laser printer cartridges – they correspond to the subtractive primaries plus black. We use this system when mixing pigments in a work of art: when complementary colors are placed adjacent to one another in a painting, they enhance one another producing a brightening effect; adjacent non-complementary colors, on the contrary, produce a duller effect (Chapter 13.4). Additive color mixing is good for adding lights such as theatre lights and the pixels on your television screen; it is also the principle behind photonic and structural colors (Chapter 16.3).
Figure 1.3Left: Additive primary color circles; Reproduced from ref. 14. Right: Subtractive color primary circles. Reproduced from https://commons.wikimedia.org/w/index.php?title=File:Subtractive_color_mixing.webp&oldid=464911059 under the terms of the CC BY-SA 3.0 license, https://crea...