1.1.1 The beginning: Just philosophy
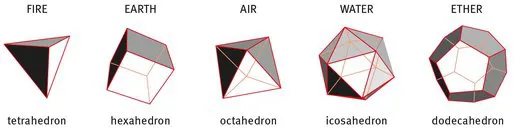
The concept of an atom was approached about 24 centuries ago, when ARISTOTLE (384–322 BC) suggested that all existing matter is built of four components, namely air, water, fire and earth. PLATO (427–347 BC) added a fifth element, the ether, as the one to allow interaction and transformation between the others. The five Platonic solids are illustrated in Fig. 1.1. Interestingly, they are each composed of just one of only three single abstract geometric figures (equilateral triangle, square and a regular pentagon), which when assembled form the tetrahedron, the hexahedron, the octahedron, the icosahedron and the additional one, the dodecahedron.
Almost at the same time, the Greek philosopher LEUCIPPUS (ca. 450–370 BC) and his scholar DEMOCRITUS (460–371 BC) proposed a material world made of indivisible components. Assuming that every material could be divided into smaller parts, and these again into even smaller fragments, finally a level is reached of no longer divisible things. They called them atomos, which is the Greek name of atoms, the indivisible. The philosophers subscribed them with defined properties, namely to be:
- – very small,
- – and thus invisible,
- – indivisible,
- – hard,
- – of different forms (however without color, taste or smell)
- – moving spontaneously and continuously (in an empty space, i.e. in vacuo or in “ether”).
Figure 1.1: The five Platonic solids. Each is composed of a number of identical isosceles surfaces: tetrahedron (4 triangles), hexahedron (6 squares), octahedron (8 triangles), icosahedron (20 triangles), dodecahedron (12 pentagons).
To conceive of the ultimate building blocks as having “different forms” included the elegant idea that the matter built of them finally reveals properties of the “atoms”, which themselves remain invisible. Ultimately, the building blocks are not only the composites of our material world – they are responsible for its properties.
1.1.2 2000 years later: Experimental evidences
It took more than 22 centuries until this conceptual idea was proven experimentally. Chemists like LAVOISIER (1743–1794), PROUST (1755–1826) and DALTON (1766–1844) realized that individual chemical elements combine mass-wise to compounds according to some rules.
Conservation of mass (LAVOISIER): The overall mass represented by all reaction partners remains constant. For a chemical reaction, the total mass is divided among all the species involved and, in the case of complete reaction, the mass of all reaction products is equal to the mass of the reactants. If hydrogen gas and oxygen gas undergo the oxyhydrogen gas reaction, e.g. 4 g of H2 react with 16 g of O2 (together making 36 g) to form 36 g of water. This experimental evidence is the law of conservation of mass (cf. Table 1.1).
Law of definite proportions (PROUST): Species undergoing a chemical reaction combine not stochastically, but in characteristic ratios. Today, we know that this is according to the number of moles, which in turn represent the number of species. In the case where the two gases hydrogen and oxygen react to form water, hydrogen and oxygen combine in molar ratios of 2 : 1. According to their individual masses, this reflects a mass ratio of 1 : 8, whatever the different amounts of the two gases of hydrogen and oxygen were at the beginning of the chemical reaction. This experimental fact constitutes the law of definite proportions (cf. Table 1.1).
Table 1.1: The laws of conservation of mass and of definite proportions exemplified for the formation of water out of the two gases hydrogen and oxygen. The ratio between the two elements is always 1 : 8 if masses are counted, and 2 : 1 if moles are considered. Using the AVOGADRO number to convert moles into numbers of atoms, it is obvious that the overall number of atoms of th...