![]()
Filaretova LP, Takeuchi K (eds): Cell/Tissue Injury and Cytoprotection/Organoprotection in the Gastrointestinal
Tract: Mechanisms, Prevention and Treatment. Front Gastrointest Res. Basel, Karger, 2012, vol 30, pp 181–190
______________________
Involvement of Renin-Angiotensin System and Vasoactive Angiotensin-(1–7) Metabolite of Angiotensin II in Gastric Mucosal Injury and Gastroprotection
Tomasz Brzozowski · Slawomir Kwiecien · Aleksandra Szlachcic · Malgorzata Strzalka · Danuta Drozdowicz · Robert Pajdo · Jakub Szmyd · Stanislaw J. Konturek · Wieslaw.W. Pawlik
Department of Physiology, Jagiellonian University Medical College, Cracow, Poland
______________________
Abstract
The renin-angiotensin system (RAS) plays an important role in the maintenance of blood pressure, cardiovascular functions, body fluids homeostasis and kidney reabsorption process but little is known whether angiotensin derivative metabolites, the classic component of the systemic and local RAS exhibit gastroprotective and ulcer healing properties. Angiotensin II (Ang II) formed from angiotensin I due to angiotensin-converting enzyme (ACE) binds to the Ang type 1 (AT1) and type 2 (AT2) receptors, both implicated in the pathogenesis of cold stress- and ischemia-reperfusion-induced gastric lesions. The new functional components of RAS, such as Ang-(1-7), Ang IV, Ang-(1-12) and novel pathways ACE2 have been recently proposed to maintain physiological functions in the gastrointestinal (GI) tract. In this review, we describe the contribution of the Ang II metabolite, Ang-(1-7), to gastroprotection against stress-induced gastric lesions. First, Ang-(1-7) is produced in excessive amounts in the gastric mucosa of rodents, suggesting that this metabolite could be involved in the mechanism of gastric mucosal defense. Second, pretreatment with Ang-(1-7) attenuated the gastric lesions induced by cold-restraint stress and raised the gastric blood flow, suggesting that this vasoactive metabolite of Ang II could be involved in the mechanism of gastric integrity and gastroprotection. This protective response can be demonstrated in experimental damage induced by acid-dependent (stress, ischemia-reperfusion) and acid-independent (ethanol) injury. We conclude that full understanding of the metabolic pathways of Ang I and Ang II conversion into vasoactive metabolites such as Ang-(1-7) in the stomach and the efficacy of Ang-(1-7) to attenuate gastric lesions induced by damaging agents may be useful in the treatment of upper GI disorders including the mechanism of protection against mucosal damage induced by various ulcerogens and in the process of ulcer healing.
Copyright © 2012 S. Karger AG, Basel
Physiology of the Renin-Angiotensin System and Angiotensin Receptors
The renin-angiotensin system (RAS) was initially discovered by Tigerstedt and Bergmann [1] in 1898 and it has now become extensively investigated. The activation of RAS remains a sequential step to the activation of the precursor molecule angiotensinogen. Due to the action of renin, angiotensin I, an inactive decapeptide, is first formed and it is then converted to its active component angiotensin II (Ang II) by a peptidase, the angiotensin-converting enzyme (ACE), which is predominantly located on the surface of lung endothelial cells. The octapeptide Ang II was discovered as a circulating pro-hypertensive principle of renal origin [2]. Originally, the circulating Ang II was considered the main effector acting via its binding to two major receptor subtypes the Ang type 1 (AT1) and type 2 (AT2) involved in the regulation of blood pressure, body fluid control, renal salt and water retention and to facilitate sympathetic transmission [3, 4]. Both AT1 and AT2 receptor subtypes may play a divergent role, because the AT1 receptor is involved in the cell proliferation and the production of cytokines, while the AT2 receptor was reported to regulate the control of blood pressure and to cause the inhibition of cell proliferation, thus contributing to neointimal formation after vascular injury [5]. Some Ang II responses, including NO release and collagen synthesis are mediated by both AT1 and AT2 receptors [6]. Both AT1 and AT2 receptor antagonists showed a satisfactory efficacy and safety allowed for widespread use in the treatment of hypertension. Furthermore, their anti-inflammatory and vascular protective effects contribute to reduction of the renal and cardiovascular failure [7]. Blockade of the AT1 receptors in humans is also neuroprotective, reducing the incidence of stroke, improving cognition and decreasing the progression of Alzheimer's disease and could be useful as therapeutic option in lung disorders such as chronic obstructive pulmonary diseases (COPD) and acute respiratory distress syndrome [8].
Importance of RAS in the Mechanism of Gastric Mucosal Integrity
RAS exerts its endocrine effect on hemodynamic regulation and body fluid homeostasis [9], but less is known about its regulatory impact of the tissue based local RAS, especially in the GI tract. The existence of local RAS has been demonstrated in gastrointestinal organs including the pancreas, esophagus and colon [10, 11].
RAS plays an important role in the mechanism of gastric integrity and gastroprotection and the pathogenesis of stress, indomethacin and ischemia-reperfusion induced gastric damage [12–15]. It is well known that stress induces acute gastric mucosal lesions by complex psychological factors influencing individual vulnerability, stimulation of specific brain pathways regulating autonomic function, decreased blood flow to the mucosa, hypermotility, mast cell degranulation, leukocyte activation, and increased free radical generation resulting in increased lipid peroxidation [16, 17] (fig. 1). Cold-restraint stress is commonly used and a clinically relevant experimental model for acute gastric damage [18]. The cessation of gastric microcirculation within gastric mucosa and increased free radical formation could be the major explanation for hemorrhagic gastric lesions and erosions predisposing to ulcer formation [17]. The maintenance of gastric blood flow is important to protect the mucosa from endogenous and exogenous damage factors. This gastric blood flow is impaired due to Ang II and catecholamines that are activated during stress in both plasma and peripheral tissues including the stomach [11]. Ang II contributes to the pathogenesis of inflammation induced by various stressors such as cold stress and ischemia-reperfusion (I/R) resulting in acute lesions in the gastric mucosa of rodents [17, 19] (fig. 1). Ang II elicits a cellular response through several molecular signaling pathways, such as calcium mobilization, reactive oxygen metabolite generation, and activation of protein kinase and nuclear transcription factors including nuclear factor-κB (NFκB) [20]. On the other hand, this peptide might contribute to the mucosal defense mechanism via activation of the vascular tone in the arteries of resistance. However, Ang II was shown to not only regulate vascular tone in the arteries of resistance and in the brain tissue but also constricts the gastric vasculature through AT1 receptor stimulation [21]. In addition, Ang II was shown to generate an excessive amount of reactive oxygen species followed by cellular damage and inflammation and to cross-talk with other inflammatory second messengers such as nitric oxide (NO), and PGs through NFκB-dependent mechanism [22, 23].
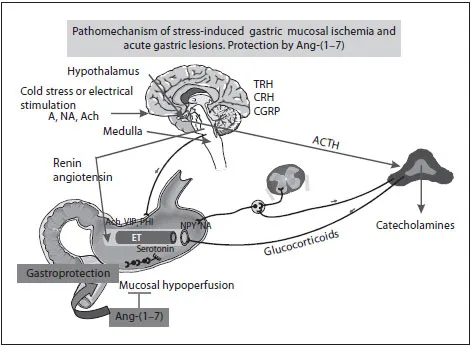
Fig. 1. Schematic representation of mechanisms involved in pathogenesis of stress-induced gastric lesions. Cold stress or electrical stimulation to hypothalamic areas cause gastric lesions due to activation of sympathetic system and catecholamines release from the adrenal medulla leading to a potent vasoconstriction in gastric microcirculation that is prerequisite for mucosal ischemia, damage and ulcer formation. RAS resulting in activation of Ang II contributes to this vasoconstrictive effect on gastric microvessels, i.e. mucosal hypoperfusion and development of gastric mucosal bleeding erosions. In contrast, the vasoactive metabolites of Ang II such as Ang-(1-7) exert a gastroprotective effect against stress-induced gastric lesions due to vasodilatation and mucosal hyperemia.
The inhibition of Ang II AT1 receptors with peripheral and central receptor antagonists prevents the sympathoadrenal and hypothalamic-pituitary-adrenal response to isolation stress and protects the brain from injury by reducing the cerebral blood flow during a stroke [19, 24]. The question remains whether antagonism of the AT1 receptors could attenuate the incidence of cold stress-induced gastric lesions and whether AT1 receptor blockade could be of therapeutic usefulness in this stress-related disorder. To address this question, a widely used strain of spontaneously hypertensive rats (SHR) characterized by increased sympathoadrenal reactivity to stress was employed because of the well-known association between stress and cardiovascular disease [11]. In this study, the AT 1 antagonists exhibited the protection against stress-induced gastric lesions due to the suppression of the stress-induced hormonal axis and sympathoadrenal response, the attenuation of the vasoconstrictor effects of Ang II in the gastric microcirculation, thereby causing an inhibition of mucosal inflammation [11, 12].
During intense acute stress, such as immobilization, there is an initial m...