![]()
Arzt E, Bronstein M, Guitelman M (eds): Pituitary Today II: New Molecular, Physiological and Clinical Aspects.
Front Horm Res. Basel, Karger, 2010, vol 38, pp 145–151
______________________
Serum Insulin-Like Growth Factor-1 Measurement in the Diagnosis and Follow-up of Patients with Acromegaly: Preliminary Data
Mirtha Guitelmana,b · Graciela Radczukc · Natalia García Basavilbasoa,b · Adriana Onetob,c · Armando Bassob
aDivisión Endocrinologia Hospital Carlos G. Durand, bInstituto de Neurocirugía de Buenos Aires (INBA), and cLaboratorio TCba Salguero, Buenos Aires, Argentina
______________________
Abstract
Measurement of serum insulin-like growth factor-1 (IGF-I) is the current method for diagnosing and monitoring acromegaly. However, the use of commercially available kits needs to be validated. In our study, we have investigated the use of two different IGF-I immunoassays in patients already diagnosed with acromegaly. We compared a two-site immunoradiometric assay with ethanol-acid extraction (IRMA-DSL) and a solid-phase chemiluminescent immunometric assay (ICMA-IMMULITE), correlating the clinical finding with the biochemical results. A total of 102 samples (77 women and 25 men aged 18-79 years) were analyzed with the two different IGF-I assays. Sixty-four of samples had been taken from patients with acromegaly in different stages. Pearson regression showed a high correlation coefficient; otherwise, Bland and Altman analyses showed a mean difference of 177.6 ng/ ml, with upper and lower limits of-183.5 and 538.7 ng/ml in the 102 samples studied. Normal serum IGF-I was found in 64 and 41.5% of patients with treated acromegaly when measured by ICMA and IRMA, respectively. In our study, IGF-I-ICMA had a better clinical correlation in patients with treated acromegaly.The reevaluation of current IGF-I immunoassays is necessary to correctly interpret treatment response in acromegalic patients and thus achieve a better correlation between clinical and biochemical results.
Copyright © 2010 S. Karger AG, Basel
Acromegaly is the condition that results from prolonged and excessive circulating growth hormone (GH) levels in adults. GH primarily assists in the synthesis of peripheral insulin-like growth factor-I (IGF-I), mostly in hepatocytes, a process that leads to cell proliferation and inhibition of apoptosis.
IGF-I is a useful tool for the diagnosis, postoperative assessment of regression or ‘cure’, and long-term monitoring of active acromegaly [1, 2]. The increased rates of morbidity and mortality associated with acromegaly make reliable methods of assessment essential [3].
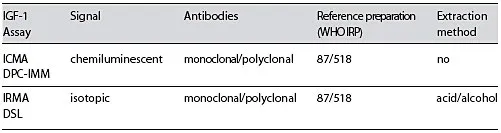
Table 1. Serum IGF-1 assays: characteristics
GH acts mostly through IGF-I. This is supported by the fact that, in patients with GH insensitivity, exogenous IGF-I promotes growth and may even mimic the phenotype of acromegaly, indicating that it is IGF-I and not GH the true growth hormone in this disease [4]. In addition, the use of the GHR antagonist Pegvisomant, which blocks signal transduction and transcription of serum IGF-I, results in resolution of symptoms, signs, and metabolic features of acromegaly, despite a persistently elevated GH[5, 6].
There are some physiological factors that change IGF-I levels, mainly age and binding proteins [7]. The IGF-I surge in puberty results from values outside ‘normal’ age-related reference ranges. IGF-I also shows an age-dependent decrease of 10-16% per decade, even in acromegaly [8]. Ninety-nine percent of the circulating IGF-I is bound together with IGFBP-3 and the acid-labile subunit (ALS) in ternary, 150-kDa complexes. This complexation prolongs the half-life (15 h) of IGF-I and prevents its transfer out of the vascular space [9, 10].
There are several problems associated with serum IGF-I measurements, including difficulty in comparing results among laboratories due to a lack of standardization, susceptibility to interference from IGF-binding proteins (IGFBP), lack of a pure international reference preparation, and the need for appropriate age-adjusted normative data. Current commercially available immunoassays differ in terms of assay principle (competitive vs. noncompetitive), assay format (manual vs. automated), type of antibodies used (monoclonal vs. polyclonal) and label used (radioactive, enzyme-linked detection or chemiluminescent detection) [11, 12].
In addition, assays can be divided, according to the method used to avoid interference from IGFBP, into nonextraction and extraction methods [13]. Such pitfalls in the fine-tuning of this hormone, together with the clinical discrepancies we found, led us to investigate two different immunoassays in patients with GH excess.
The most widely used immunoassay in our country until 2007 was the two-site immunoradiometric assay with ethanol-acid extraction, IRMA-DSL. With regard to patient follow-up during treatment, we found discrepancies between the clinical score and biochemical results. Then we decided to include a new immunoassay in order to establish the cause of those discrepancies.
Objective
In our study, we investigated and compared two different commercially available IGF-I immunoassays in patients with known acromegaly, and correlated their clinical finding and biochemical results. We also included a small group of normal subject as control.
Patients and Methods
Subjects and Patients
A total of 102 samples (77 women and 25 men, 18-79 years old) were analyzed using two different IGF-I assays. Sixty-four of the samples had been taken from acromegalic patients, 44 of which were under different medical treatments (35 were receiving somatostatin analogs, 5 pegvisomant, and 4 cabergoline), 11 had active disease and were under no medical treatment, and 9 were patients who had attained postoperative cure (1 had undergone radiation therapy). Four additional samples had been taken from 4 subjects diagnosed with acromegaly due to high levels of IGF-I measured by IRMA; all of them were asymptomatic, showed a normal pituitary MRI, and had GH levels <1 ng/ ml in the oral glucose tolerance test (OGTT).
Acromegaly was diagnosed on account of the findings of both lack of GH suppression during OGTT (GH >1 µg/l) and higher than normal age-matched serum IGF-I levels, along with clinical features of GH excess and pathological MRI findings [14]. Acromegaly is considered to be controlled or cured based on biochemical parameters, including GH levels lower than 1 ng/ml in the OGTT and normal age-matched IGF-I [14–15].
Our control group consisted of 38 samples from nonacromegalic patients.
Laboratory Methods
Assay 1. Two-site immunoradiometric assay with ethanol-acid extraction, IRMA-DSL.
Assay 2. Solid-phase chemiluminescent immunometric assay, ICMA-IMMULITE.
Reference ranges for each method were used as provided by the manufacturers. Immunoassays characteristics are shown in table 1.
Statistical analyses were performed by Pearson correlation and Bland-Altman tests.
Results
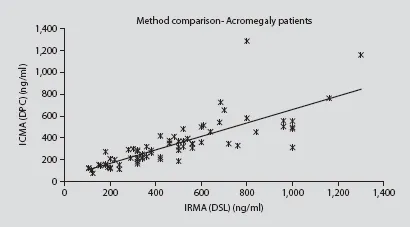
Pearson regression showed a correlation coefficient of r = 0.8165 (significance level p< 0.0001; fig. 1). Bland and Altman analyses showed a mean difference of 177.6 ng/ ml, with upper and lower limits of-183.5 and 538.7 ng/ml in the 102 samples studied (table 2).
Fig. 1. Pearson correlation between ICMAand IRMAof all samples analyzed.
Normal serum IGF-I was found in 64% and 41.5% of patients with treated acromegaly (surgery, medical treatment or both) when measured by ICMA and IRMA, respectively. Four patients, considered as cured, with GH levels <1 ng/ml in the OGTT, normal MRI findings, and no symptoms or signs of active disease, showed high IRMA, but normal ICMA IGF-I levels.
When patients receiving somatostatin analogs were analyzed separately, IGF-I was found to be within the normal ranges in 58% (ICMA) and 32% (IRMA).
Improvement of the clinical score was seen in 79% of all treated patients and in 77% of patients under octreotide. Four subjects who had high levels of IRMA-IGF-I but no other suspicious findings of acromegaly showed a normal ICMA-IGF-I, in accordance with their clinical status and the lack of other markers of acromegaly (GH levels <1 µg/l in the OGTT and normal MRI findings).
The 4 additional patients suspected to have acromegaly had normal ICMA IGF-I.
Discussion
Measurement of circulating total IGF-I levels provides an important tool for diagnosing GH disorders as well as for monitoring treatment efficacy; however, several problems can diminish its clinical usefulness. Serum IGF-I levels vary depending on biological determinants and assay performance. This variability may have its physiological source in features such as nutritional status, pubertal stage, pregnancy, age, and gender, all of which should be taken into account when interpreting IGF-I values [16, 17].
Our f...