![]()
1 The Nature of Silver
Silver is a siren. It is the earth’s most shiny, reflective metal, spinning light into our eyes like a flipped silver dollar, or drachma, or peso, blinding us to its corruptive potential and hurling men and empires (and vampires) to ruin. We have coveted it – sometimes over its rarer and costlier cousin, gold – since we first learned how to extract it from the earth. It has been awarded to our victors: at the first modern Olympic Games in Athens, in 1896, the winners were crowned with an olive wreath and awarded a silver medal; the ‘gold’ medals of subsequent first-place winners have almost always been gold-plated silver.1 Silver brings out our most generous instincts – as, for example, when we give it as a gift to celebrate a marriage, birth or special achievement – and our very worst. A highly publicized wrangle over ownership of the ‘Sevso Treasure’, a spectacular hoard of exquisitely crafted late Roman silver, involved three alleged murders before some of it was repatriated by the Hungarian government in 2014.
All this, of course, has more to do with human nature than the nature of silver. But what is it about silver that incites these sentiments and actions? After all, just like gold, diamonds, emeralds – all of the precious metals and gems for which we profess love and wage war – silver started out as space debris. The question of why we value some things over others has exercised philosophers for millennia. As the fourth-century Christian leader John Chrysostom so profoundly enquired of his increasingly materialistic flock, ‘Are gold and silver beautiful? But consider that they were and still are dirt and ashes.’2 Chrysostom was urging the errant Christians of late Antiquity to accumulate spiritual rather than material capital, but his reference to filth was double-edged. While coveting earthly treasure might be degrading, it is also nonsensical – if everything has the same origin and comes to the same end, why value silver over, say, zinc?
Silver structure
Perhaps if we take a closer look than Chrysostom was able to, we might understand silver’s allure a little better. What we currently understand about silver’s structure and behaviour in relation to other substances can help explain the properties we prize, though knowledge shifts constantly, especially of a material so diversely useful to contemporary life. Silver has the atomic number 47, with 47 positively charged protons in its nucleus, and 47 negatively charged electrons around the nucleus. These atoms pack together to form crystals in an orderly system we call metallic bonding. When metal atoms come together, most of the electrons are held tight by the positively charged nuclei, but some detach and join a ‘sea of electrons’ – a fluid environment for the flow of electrons that is an electrical current. Think of a riverbed that throws a few obstacles (rocks, roots, twists or tributaries) into the course of water. One of the reasons we value silver today is that, of all metals, it is the most effective conductor of electricity, and is used in high-performance electronics and electrical systems. The structure of silver creates an environment most conducive to this uninterrupted flow, though for reasons of cost, its close competitor copper is more widely used for everyday needs such as electrical wiring.
The malleability of silver – the extent to which it may be moulded to a different shape – means that it can be hammered into flat sheets, or formed into seamless round vessels. Some metals, such as titanium and steel, require heat to become malleable. Others, like gold and silver, are much softer and can be partially worked cold. These softer metals tend to have weaker metallic bonding, allowing the metal atoms to slide against each other under pressure. Similarly, weaker bonding results in gold and silver being ductile, characterized as being capable of being drawn into wires – a property exploited by silversmiths who have created delicate filigree ornament of thread-fine silver.
The symbol for silver on the periodic table of the elements is Ag, from the Latin argentum, whose root in both Latin and Greek means white or shining. Silver’s perfect sheen comes from the fact that it is the most reflective of all metals. Over the light spectrum visible to humans (between about 400 and 700 nm), which we perceive as our gloriously multicoloured world, silver trumps all other metals in its constant reflectivity. Again, this has much to do with those free-flowing electrons. As waves of incoming light hit the electrons on the metallic surface, the agitated electrons create their own opposing field of energy that is pushed outwards in another wave – a reflection. This occurs in silver across all the wavelengths we can see, resulting in the reflection of clean and even light that we put to many uses. High-quality mirrors were historically coated with silver, and it is still used in scientific mirrors, precision optical equipment and telescopes.
Reliquary cross, Venice, c. 1750–1800, silver. | |
Silver, along with gold, platinum and a small number of other metals, is classified as a noble metal, which means that it resists oxidation. Unlike iron, for example, it does not rust in contact with moisture-laden air or water. It does not readily react with oxygen in the air, though unlike gold, it does tarnish when exposed to sulphur compounds. The more heavily polluted the air, the quicker the build-up of black silver sulphide. A surprising consequence of living in the industrialized world is the need to frequently polish silver jewellery and tableware.
While family silver may not be much of a preoccupation of our contemporary world, advances in health care and infection control are. For centuries silver has been valued as a cleansing agent to purify water or disinfect wounds, but it is only recently that we have begun to understand just how silver destroys bacteria. Its antimicrobial properties are only activated through chemical reaction in silver’s ionized form – that is, when the atoms, having lost an electron, become positively charged. Like all things organic, bacteria rely on enzymes to sustain life and flourish. It is these enzymes that silver ions attack and disable. In short order, the bacteria cells dehydrate, shrivel and die. An unexpected bonus is that the silver-impregnated dead cells ‘infect’ their healthy neighbours, leading to a wide-scale sustained massacre dubbed the ‘zombies effect’ by the scientists who recorded it.3
Origins
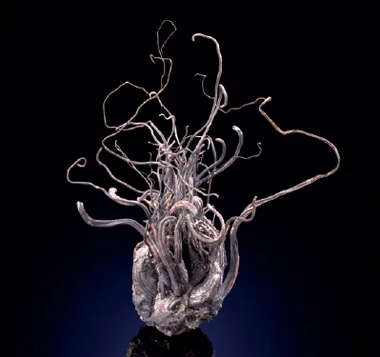
Among the breathtaking display of minerals and gemstones at the Freudenstein Castle in the historic mining town of Freiberg, Germany, is a specimen of silver. At a height of 15 cm (6 in.), it looks like an intricately twisted wire sculpture, or a sinuous underwater plant. Gorgeous specimens of pure or ‘native’ silver, looking like cunningly pruned bonsai trees, or delicate fronds of coral, belong to prestigious mineral collections throughout the world. Readily collectible smaller versions are regularly offered by dealers. Finger-length pieces, approximately 8 cm (3 in.), might cost the same as a luxury car. As a centre of silver mining for eight hundred years, the region around Freiberg was richly productive. One mine produced a single specimen weighing 225 kg (500 lb) in 1857.4
Native wire silver with acanthite from the Himmelsfurst Mine, Brand-Erbisdorf, Freiberg District, Erzgebirge, Saxony, Germany. | |
Silver is widely dispersed around the globe, and specimens of great substance and beauty have been found in places ranging from Alaska to Australia to China – the last a new source for small collectible pieces. Britain, too, had silver mines. The most productive were in Cornwall, but Scotland had a small eighteenth-century mine with rich native silver deposits at Alva, near Stirling, where delicate tree-like crystals were retrieved in the 1980s. Some of the most intricate and sought-after specimens originated in Kongsberg, Norway, which produced large masses of lustrous wire, some a metre (3 ft) long. In terms of heft, however, these cannot compare with the slabs of silver discovered near Sonora, Mexico, or the sheets mined in Cobalt, Ontario, in the early twentieth century.5
| Dendrites of native silver, specimen from the Alva silver mine, Clackmannanshire, Scotland. |
| An entrance to the former Alva silver mine in Scotland. |
Most mined silver does not look like this, though. Native silver is rare; it does not coil from clefts in exposed rock or gleam like gold in flecks on riverbeds. It is much more likely to exist in complicated mineral structures, locked into combinations with other elements such as lead, copper, chlorine or arsenic. We call these minerals ‘ores’ if they contain a valuable constituent (such as silver) that can be extracted for profit. Most silver-mining museums have samples of ores that to the untrained eye look unprepossessing, or even deceptive. For instance, a chunk of acanthite in quartz looks lumpen and sooty grey; proustite glints ruby-red. The story of how silver arrived in this form gives us a framework for understanding how we recognize it as silver, and retrieve it from the ground.
All earth’s elements except the lightest, such as hydrogen and helium, were formed inside stars as they evolved, or at the point of their death. As stars burn through their fuel, lighter elements combine to create heavier ones, such as carbon, in a process known as fusion. Heavy metals like silver and gold, though, require an even more intense crucible in order to form – the death of the star itself, hurling elements into space as it explodes. The silver on our planet was forged from the supernovae of stars eight to nine times the mass of our sun. The stars that birthed gold were even larger, so although gold and silver are sometimes found together in nature, and although our culture often pairs them, their parents were quite separate.6
Fast forward – way forward – through the creation of the Earth and the tumultuous metamorphoses of its crust. One of the most common ways that silver concentrates near the surface where we can find it is through the action of hot fluids. These metals that were once space refuse dissolve in magma-heated water circulating deep within the earth’s crust. This vast hydro-thermal plumbing system carries the mineral-charged solutions up into fissures and faults in rocks. As they cool, or react chemically with the rocks, the minerals are deposited. Sometimes they are exposed by erosion at the earth’s surface; sometimes their discovery is more challenging. Silver starts out in space, but where it ends up is very much a matter of local conditions near the earth’s surface, dependent on the pattern of faults and types of rocks.
Treasure hunt
Today throughout the world most silver is produced as a by-product of mining for other metals such as copper or lead. There are, however, substantial mines whose primary product is silver, and multinational mining companies that focus on discovery and extraction of silver. Discovering silver is both an art and a science, as no two deposits are exactly alike, and the veins, in relation to their surroundings, are vanishingly small. Although silver is more common than gold, its occurrence in the earth’s crust is only a scant 0.07 parts per million.7 The era of stumbling upon silver-rich outcrops is long gone.
Investigation into the economic viability of a specific piece of land for mining is termed exploration. Although large mining companies may conduct exploration with an in-house team of geologists and geophysicists, today a more common model is for small junior mining companies to conduct the primary research based on their preliminary identification of promising regions. Much of their work – mapping, sampling and drilling – is conducted on the ground. If a junior mining company succeeds in identifying a potential deposit, it typically sells its findings to a larger corporation which would take the project through development.8 It is a h...