
Introduction to Applied Colloid and Surface Chemistry
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Introduction to Applied Colloid and Surface Chemistry
About this book
Colloid and Surface Chemistry is a subject of immense importance and implications both to our everyday life and numerous industrial sectors, ranging from coatings and materials to medicine and biotechnology.
How do detergents really clean? (Why can't we just use water?) Why is milk "milky"? Why do we use eggs so often for making sauces? Can we deliver drugs in better and controlled ways? Coating industries wish to manufacture improved coatings e.g. for providing corrosion resistance, which are also environmentally friendly i.e. less based on organic solvents and if possible exclusively on water. Food companies want to develop healthy, tasty but also long-lasting food products which appeal to the environmental authorities and the consumer. Detergent and enzyme companies are working to develop improved formulations which clean more persistent stains, at lower temperatures and amounts, to the benefit of both the environment and our pocket. Cosmetics is also big business! Creams, lotions and other personal care products are really just complex emulsions.
All of the above can be explained by the principles and methods of colloid and surface chemistry. A course on this topic is truly valuable to chemists, chemical engineers, biologists, material and food scientists and many more.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
Introduction to Colloid and Surface Chemistry
1.1 What are the colloids and interfaces? Why are they important? Why do we study them together?
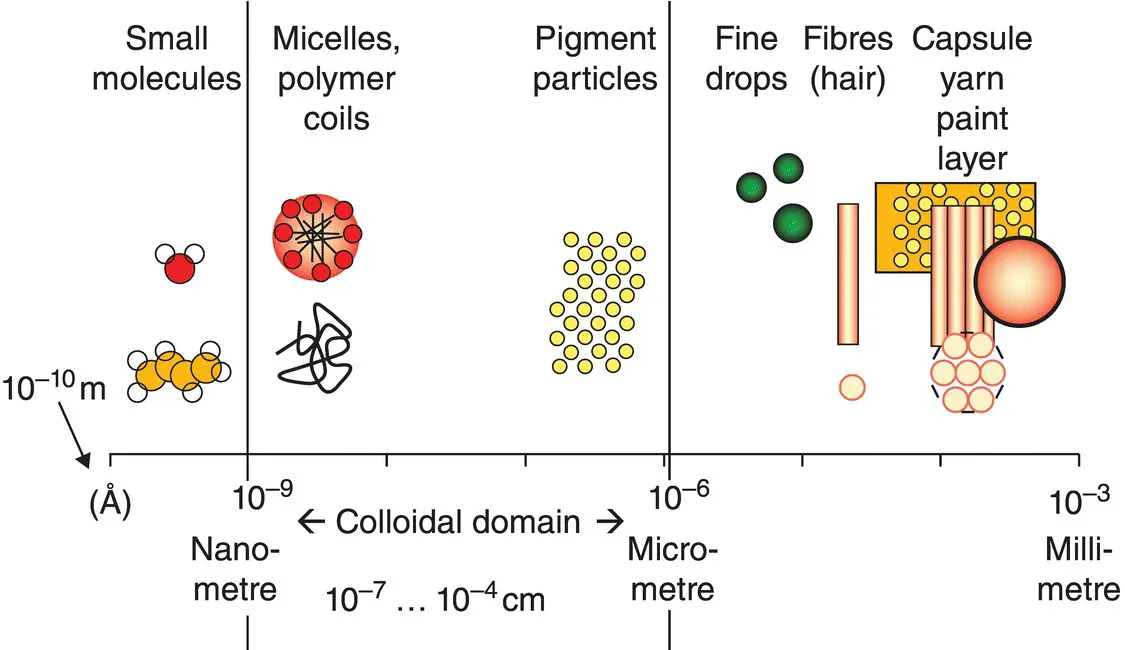


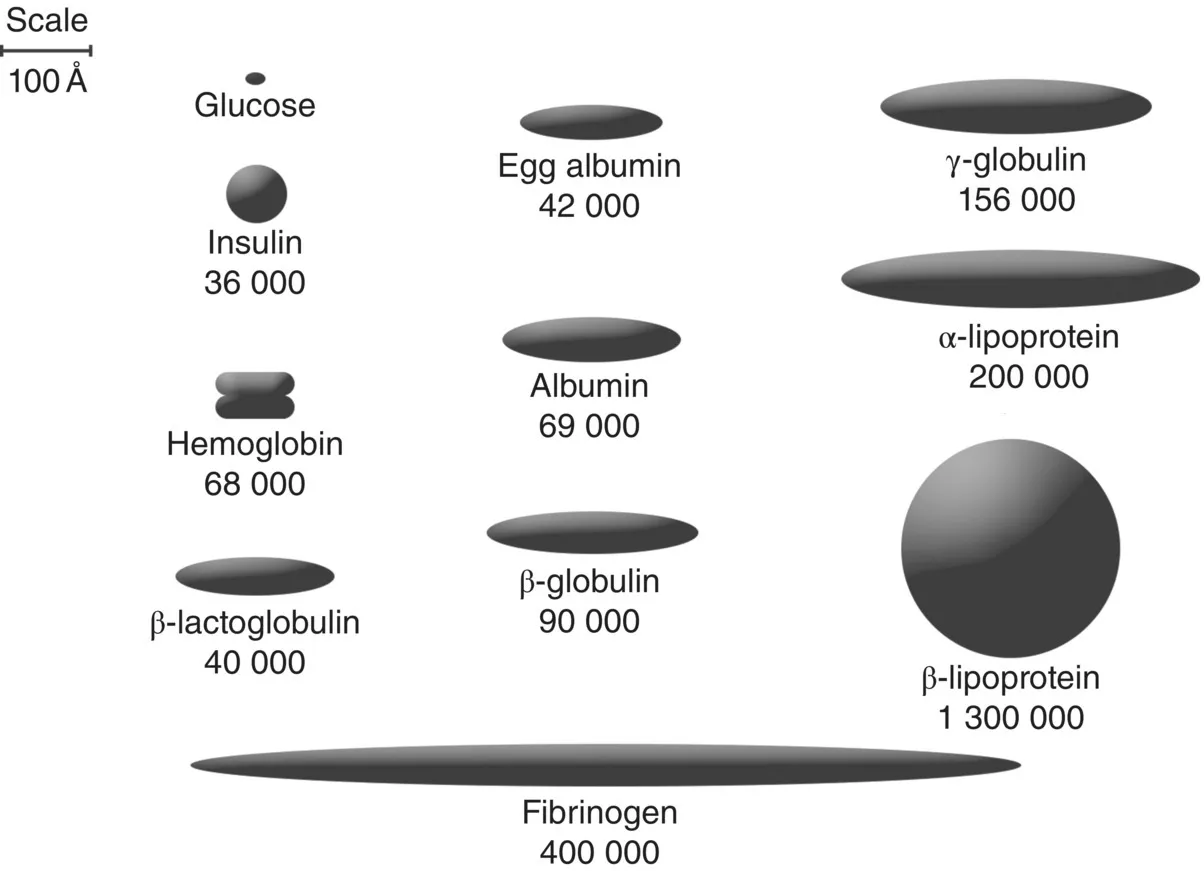
1.1.1 Colloids and interfaces
Table of contents
- Cover
- Title Page
- Table of Contents
- Preface
- Useful Constants
- Symbols and Some Basic Abbreviations
- About the Companion Web Site
- 1 Introduction to Colloid and Surface Chemistry
- 2 Intermolecular and Interparticle Forces
- 3 Surface and Interfacial Tensions – Principles and Estimation Methods
- 4 Fundamental Equations in Colloid and Surface Science
- 5 Surfactants and Self-assembly. Detergents and Cleaning
- 6 Wetting and Adhesion
- 7 Adsorption in Colloid and Surface Science – A Universal Concept
- 8 Characterization Methods of Colloids – Part I
- 9 Characterization Methods of Colloids – Part II
- 10 Colloid Stability – Part I
- 11 Colloid Stability – Part II
- 12 Emulsions
- 13 Foams
- 14 Multicomponent Adsorption
- 15 Sixty Years with Theories for Interfacial Tension – Quo Vadis?
- 16 Epilogue and Review Problems
- Index
- End User License Agreement
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app