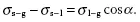Everyone talks about nanomaterials. There are many publications, books and journals devoted to this topic. This is not surprising, as the economic importance is steadily increasing. Additionally, interested persons without specific education in one of these fields, have, at the moment, nearly no chance to understand this technology, its background and applications. This book fills this gap. It deals with the special phenomena found with nanomaterials and tries to give explanations, avoiding descriptions that are directed to specialists and need specialized education.
To get an idea about the actual size relations, think about a tennis ball, having a diameter of a little more than 6 cm = 6 × 10−2 m and compare it with a particle with diameter of 6 nm = 6 × 10−9 m. The ratio of the diameters of these two objects is 107. An object 107 times larger than a tennis ball has a diameter of 600 km. This simple comparison makes clear: nanoparticles are really small.
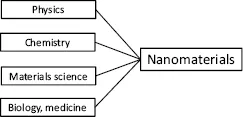
The difficulty with nanomaterials arises from the fact that – in contrast to conventional materials – knowledge of material science is not sufficient; rather some knowledge of physics, chemistry, and materials science is necessary. Additionally, as many applications are in the fields of biology and medicine, some knowledge in these fields is necessary to understand these important applications. Figure 1.1 demonstrates that science and technology of nanomaterials are influenced by materials science, physics, chemistry, and for many, economically most important applications, also of biology and medicine.
The number of additional facts introduced to materials science is not that large, therefore, this new situation is not that complicated, as it may look to the observer from the outside. However, the industrial user of nanomaterials, as a developer of new products, has to accept that the new properties of nanomaterials demand deeper insight to the physics and chemistry of the materials. Furthermore, in conventional materials, the interface to biotechnology and medicine depends on the application. This is different in nanotechnology, as biological molecules, such as proteins or DNAs are building blocks, quite often also for applications outside of biology and medicine.
The first question to be answered is: What are nanomaterials? There are two definitions. One, the broadest, says: nanomaterials are materials with sizes of the individual building blocks below 100 nm at least in one dimension. This definition is quite comfortable, as it does not require deeper thoughts about properties and applications. The second definition is more restrictive. It says that nanomaterials are ones with properties inherently depending on the small grain size. As nanomaterials usually are quite expensive, such a restrictive definition makes more sense.
The main difference between nanotechnology and conventional technologies is the “bottom-up” approach preferred in nanotechnology, whereas conventional technologies usually prefer the “top-down” approach. The difference between these two approaches is explained for example, using the example of powder production. In this context, chemical synthesis is typical of the “bottom-up” approach; whereas, crushing and milling are techniques that may be classified as “top-down” processes. Certainly, there are processes, which may be seen as “in between”. A typical example is the defoliation of silicates or graphite to obtain graphene.
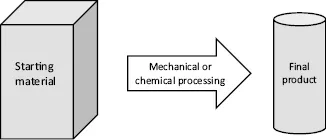
The expression “top-down” describes processes starting from large pieces of material to produce the intended structure by mechanical or chemical methods. As long as the structures are in a range of sizes accessible by mechanical tools or photolithographic processes, top-down processes have an unmatched flexibility in application. Figure 1.2 summarizes the basic features of top-down processes.
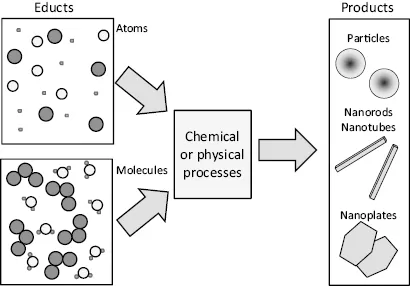
“Bottom-up” processes are, in general chemical processes starting from atoms or molecules as building blocks to produce nanoparticles, nanotubes or nanorods, thin films or layered structured. Using their dimensionality for classification, these features are also called zero-, one-, or two-dimensional nanostructures. This is graphically demonstrated in Figure 1.3. Bottom-up processes give tremendous freedom in the composition of the resulting products; however, the number of possible structures to be obtained is comparatively small. Ordered structures are obtained by processes that are supplemented by self-organization of individual particles. Often, top-down technologies are described as subtractive ones, in contrast to additive technologies describing bottom-up processes.
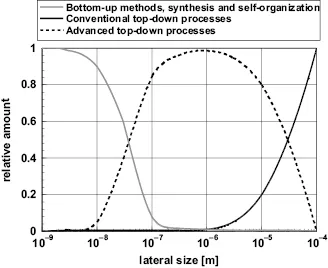
Figure 1.4 shows the size ranges of the different processes applied in nanotechnology. Certainly, there is a broad range of overlapping, between the top-down and bottom-up technologies. Most interesting, there are improved top-down technologies, such as electron beam or X-ray lithography entering the size range typical for nanotechnologies. These improved top-down technologies obtain increasing importance, for example, in highly integrated electronic devices.
For industrial applications, the most important question is the price of the product in relation to the properties. As far as the properties are comparable, in most cases, nanomaterials and products applying nanomaterials are significantly more expensive than conventional products. This becomes problematic in cases where the increase in price is more pronounced than the improvement of the properties due to the application of nanomaterials. Therefore, economically interesting applications of nanomaterials are found primarily in areas where properties that are out of reach for conventional materials are demanded. Provided this condition is fulfilled, the price is no longer that important. However, in cases where nanomaterials are in direct competition to well-established conventional technologies, the price is decisive. This fierce price competition is extremely difficult for a young and expensive technology and may lead sometimes to severe, financial problems for newly founded companies. As a general rule one may say that in the case of nanomaterials one is rather selling “knowledge” than “tons”.
Nanoparticles are neither new nor unnatural. In nature, for example, some birds and mammals apply magnetic nanoparticles for navigation, a sense called magnetoception. In plants the phenomenon of self-cleaning of leafs caused by nanoparticles at the surface, called the Lotus effect is well known and meanwhile technically exploited for self cleaning windows or porcelain ware for sanitary use (see Box 1.1). Man-made nanomaterials, in this case nanocomposites, have been known for more than 2500 years. The Sumerians already produced a red pigment to decorate their pottery. This pigment consisted of gold nanoparticles embedded in a glass matrix stabilized with tin oxide. In science, especially chemistry, suspensions of nanoparticles have been well known since the nineteenth century; however, at that time, this science was called colloid chemistry.
Box 1.1 The Lotus Effect
As an example of a macroscopically observable phenomenon caused by nanostructures, the Lotus effect will be explained. It is well known that the leaves of the Lotus plant are always clean. This is caused by the fact that the lotus leaf can not be moistened, it is hydrophobic, each drop of water flows immediately off the leaf, picking up any dust, which is, in general, hydrophilic.
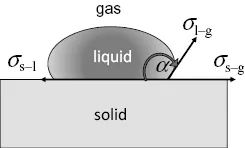
The Lotus effect is caused by an apparent increase of the contact angle between water and a solid surface. The undisturbed situation is depicted in Figure 1.5.
The contact angle, in the case of a water/solid interface, at a maximum of 110° is a result of the equilibrium of the surface stresses.
The quantities in Eq. (1.1), σs−g describe the surface stresses at the interface between the solid and the gas phase, σs−1 the surface stress between the solid and the liquid phase, σ1−g the one between liquid and the gas pas phase, and α is the contact angle. (To be mathematically exact, the surface stress is described by a vector in the tangential plane of the particle. However, for these simplified considerations, it is correct to work with the absolute values of these vectors.)
Assuming a corrugated surface with nanoparticles, as depicted in Figure 1.6, the situation conveys the impression of a larger contact angle. However, this is not correct, as the contact angle to each one of the nanoparticles has the correct value.