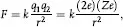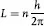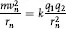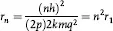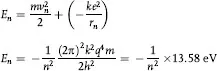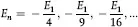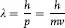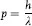![]()
1
Structure of Atoms
“I have discovered something
very interesting.”
W. C. Roentgen (Nov. 8, 1895)
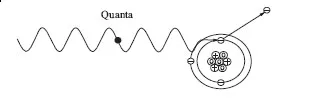
The fifty years following Roentgen’s discovery of x-rays saw remarkable changes in physics that literally changed the world forever, culminating in a host of new products from nuclear fission. Discovery of the electron (1897) and radioactivity (1898) focused attention on the makeup of atoms and their structure as did other discoveries. For example, in 1900, Planck introduced the concept that the emission (or absorption) of electromagnetic radiation occurs only in a discrete amount where the energy is proportional to the frequency, v, of the radiation, or
E = hv
where h is a constant (Planck’s constant) of nature; its value is
h = 6.1260693 × 10–34 J s or 4.13566743 × 10–15 eV s
Planck presumed that he had merely found an ad hoc solution for blackbody radiation, but in fact he had discovered a basic law of nature: any physical system capable of emitting or absorbing electromagnetic radiation is limited to a discrete set of possible energy values or levels; energies intermediate between them simply do not occur. Planck’s theory was revolutionary because it states that the emission and absorption of radiation must be discontinuous processes, i.e. only as a transition from one particular energy state to another where the energy difference is an integral multiple of hv. This revolutionary theory extends over 22 orders of magnitude from very long wavelength radiation such as radio waves up to and including high energy gamma rays. It includes the energy states of particles in atoms and greatly influences their structure.
Einstein (in 1905) used Planck’s discrete emissions (or quanta) to explain why light of a certain frequency (wavelength) causes the emission of electrons from the surface of various metals (the photoelectric effect). Light photons clearly have no rest mass, but behaving like a particle, a photon can hit a bound electron and “knock” it out of the atom.
The kinetic energy of the ejected electrons is KE = hv – φ.
Example 1–1. Light with a wavelength of 5893 Å produces electrons from a potassium surface that are stopped by 0.36 volts. Determine: a) the maximum energy of the photoelectron, and b) the work function.
Solution. a) The maximum energy KEmax of the ejected photoelectrons is equal to the stopping potential of 0.36 eV.
b) the work function is the energy of the incident photon minus the energy given to the ejected electron, or
φ = [4.13566743 × 10–15 eV s) (3 × 108 m/s) / 5.893 × 10–10 m] – 0.36 eV = 1.7454 eV.
A. H. Compton used a similar approach to explain x-ray scattering as interactions between “particle-like” photons and loosely bound (or “free”) electrons of carbon (now known as the Compton effect). Energy and momentum are conserved and the calculated wavelength changes agreed with experimental observations.
1.1
Atom Constituents
Atoms consist of protons and neutrons (discovered in 1932 by Chadwick) bound together to form a nucleus which is surrounded by electrons that counterbalance each proton in the nucleus to form an electrically neutral atom. Its components are: a) protons which have a reference mass of about 1.0 and an electrical charge of +1; b) electrons which have a mass about 1/1840 of the proton and a (–1) electrical charge; and c) neutrons which are electrically neutral and slightly heavier than the proton. The number of protons (or Z) establishes the identity of the atom and its mass number (A) is the sum of protons and neutrons (or N) in its nucleus. Electrons do not, and, according to the uncertainty principle, cannot exist in the nucleus, although they can be manufactured and ejected during radioactive transformation. Modern theory has shown that protons and neutrons are made up of quarks, leptons, and bosons (recently discovered), but these are not necessary for understanding atoms or how they produce radiant energy.
Four forces of nature determine the array of atom constituents. The electromagnetic force between charged particles is attractive if the charges (q1 and q2) are of opposite signs (i.e. positive or negative); if of the same sign, the force F will be repulsive and quite strong for the small distances between protons in the nucleus of an atom. This repulsion is overcome by the nuclear force (or strong force) which is about 100 times stronger; it only exists in the nucleus and only between protons and neutrons (there is no center point towards which nucleons are attracted). The weak force (relatively speaking) has been shown to be a form of the electromagnetic force; it influences radioactive transformation; and the gravitational force, though present, is negligible in atoms.
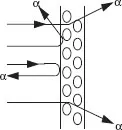
The nucleus of an atom containing Z protons is essentially a charged particle (with charge Ze) that attracts an equal number of electrons that orbit the nucleus some distance away. Thomson theorized that each negatively charged electron was offset by a positively charged proton and that these were arrayed somewhat like a plum pudding to form an electrically neutral atom. This model proved unsatisfactory for explaining the large-angle scattering of alpha particles by gold foils as observed by Rutherford and Geiger-Marsden. Such large deflections were due the electromagnetic force between a positively charged nucleus (Ze) at the center of the atom and that of the alpha particle (2e).
The force for such deflections is inversely proportional to the distance r between them, or
which yields a value of r of about 10–15 m which Rutherford proposed as the radius of a small positively-charged nucleus surrounded by electrons in orbits about 10–10 m in size. This model had a fatal flaw: according to classical physics the electrons would experience acceleration, v2/r, causing them to continuously emit radiation and to quickly (in about 10–8 s) spiral into the nucleus.
In 1913, Niels Bohr explained Rutherford’s conundrum by simply declaring (postulate I) that atoms are stable and that an electron in its orbit does not radiate energy, but only does so when it experiences one of Planck’s quantum changes to an orbit of lower potential energy (postulate III) with the emission of a photon of energy
hv = E2 – E1
And, that the allowed stationary states for orbiting electrons (postulate II) are those for which the orbital angular momentum, L, is an integral multiple of h/2π, or:
where n = 1, 2 ,3, 4, …, represents the principal quantum number for discrete, quantized energy states. Since L = mvr, the calculated radius of the first (or when n = 1) electron orbit for hydrogen (the simplest atom) was found to be 0.529 × 10–8 cm, which agreed with experiment. Postulate III is apparently based on Planck’s quantum hypothesis, but postulate II appeared to be arbitrary even though it worked, at least for hydrogen. It would only be explained by de Broglie’s hypothesis some 13 years later (see below).
Bohr assumed that electrons orbiting a nucleus moved in circular orbits under the influence of two force fields: the coulomb attraction (a centripetal force) provided by the positively charged nucleus and the centrifugal force of each electron in orbital motion at a radius, rn, and velocity, mn. These forces are equal and opposite each other, or:
where rn can be calculated from postulate II. And, since q1 and q2 are unity for hydrogen
where n = 1,2,3,4, … and r1 is the radius of the first orbit of the electron in the hydrogen atom, the so-called Bohr orbit. And since the quantum hypothesis limits values of n to integral values, the electron can only be in those orbits which are given by:
rn = r1, 4r1,9r1,16r1 …
These relationships can be used to calculate the total energy En of an electron in the nth orbit where the sum of its kinetic and potential energy is
which is the binding energy of the electron in hydrogen and is in perfect agreement with the measured value of the energy required to ionize hydrogen. For other values of n, the allowed energy levels of hydrogen are:
where E1 = –13.58 eV. These predicted energy levels can be used to calculate the possible emissions (or absorption) of electromagnetic radiation and their wavelengths for hydrogen. When Bohr did so for n = 3 he obtained the series of wavelengths measured by Balmer, and since the theory holds for n = 3, Bohr postulated that it should also hold for other values of n, and the corresponding wavelengths were soon found providing dramatic proof of the theory.
In 1926, Louis de Broglie postulated that if Einstein’s and Compton’s assignment of particle properties to waves was correct, why shouldn’t the converse be true; i.e., that particles have wave properties such that an electron (or a car for that matter) has a wavelength, associated with its motion, or
and that as a wave it has momentum, p, with the value:
This simple but far-reaching conc...