![]()
Chapter 1
Principles of bone biology and regeneration
Hector F. Rios DDS, PhD and William V. Giannobile DDS, DMedSc
Introduction
Bone is a dynamic tissue sensitive to a variety of factors with an inherent capacity that allows the translation of mechanical stimuli into biochemical signals, which therefore enhances its ability to adapt and sustain the physiological needs of the osseous structure. (Bonewald and Johnson, 2008; Burger et al., 1995; Duncan and Turner, 1995; Marotti, 2000; Marotti and Palumbo, 2007) This adaptive potential is the result of tightly regulated and synergistic anabolic and catabolic events that lead to proper metabolic and skeletal structural homeostasis (Turner and Pavalko, 1998). Multiple factors exert an effect in this system (e.g., biochemical, hormonal, cellular, biomechanical) that will collectively determine bone quality (Ammann and Rizzoli, 2003; Ma et al., 2008; Wallach et al., 1993). Clinically, bone quality is perceived as an important feature that dictates the mechanical properties of bone over time. Within the skeleton, such characteristics vary from one area to another and are determined by, among many things, cellular density and connectivity, bone density, bone macro- and microarchitecture, and the proportions of organic and inorganic matrix (Ammann and Rizzoli, 2003; Dalle Carbonare and Giannini, 2004; Ma et al., 2008; O’Brien et al., 2005; Viguet-Carrin et al., 2006). Therefore, the success of implant therapy is influenced by the understanding of the basic biological and physiological principles of bone, as it will aid the surgeon in selecting the appropriate techniques to enhance the peri-implant bone homeostasis.
Thus, the purpose of this chapter is to provide the clinician with foundational knowledge of bone development, composition, metabolism, and regeneration that serves as a primer for implant site development.
Bone Development
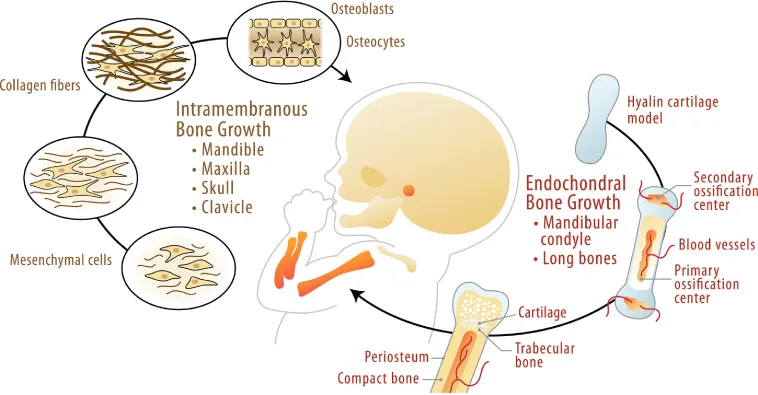
During embryogenesis, the skeleton forms by either a direct or indirect ossification process. In the case of the mandible and the maxilla, mesenchymal progenitor cells condensate and undergo direct differentiation into osteoblasts, a process known as intramembranous osteogenesis. In contrast, in the mandibular condyle, the long bones and vertebrae form initially through a cartilage template, which serves as an anlage that is gradually replaced by bone. The cartilage-dependent bone formation and growth process is known as endochondral osteogenesis (Ranly, 2000) (Fig. 1.1).
Alveolar bone lost as a result of an injury, disease, or trauma undergoes a repair process that is essentially a combination of endochondral and intramembranous complementary osteogenic processes (Rabie et al., 1996; Virolainen et al., 1995). A similar process occurs in most of the bone-related implant site development techniques, where osteoconduction, osteoinduction, and osteogenesis are exploited.
Bone Cells
Within bone, different cellular components can be identified. The distinct cell populations include osteogenic precursor cells, osteoblasts, osteoclasts, osteocytes, and hematopoietic elements of bone marrow. The content of this chapter will focus on the three main functional cells that are ultimately responsible for the proper skeletal homeostasis.
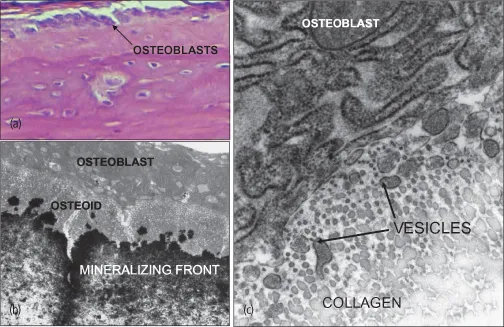
Osteoblasts are ultimately the cells responsible for bone formation. They synthesize the organic matrix components and mediate the mineralization of the matrix (Fig. 1.2). Osteoblasts are located on bone surfaces exhibiting active matrix deposition, and as their bone forming activity nears completion, some osteoblasts further differentiate into osteocytes, while others remain in the periosteal or endosteal surface of bone as lining cells. Bone lining cells are elongated cells that cover a surface of bone tissue and exhibit no synthetic activity (Rodan, 1992).
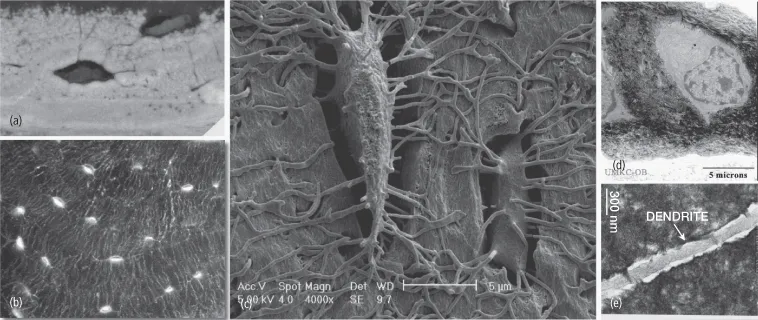
Osteocytes are stellate-shaped cells that are trapped within the mineralized bone matrix in spaces known as lacunae. They maintain a network of cytoplasmic processes known as dendrites. These osteocyte cytoplasmic projections extend through cylindrical encased compartments commonly referred to as canaliculi (Bonewald, 2007). They reach to different areas and contact blood vessels and other osteocytes. The osteocyte network is therefore an extracellular and intracellular communication channel that is sensitive at the membrane level to shear stress caused by the direction of fluid within the canaliculi space as the result of mechanical stimuli and bone deformation (Fig. 1.3). The mechanical signals are translated into biochemical mediators that will assist with the orchestration of anabolic and catabolic events within bone. This arrangement allows osteocytes to (i) participate in the regulation of blood calcium homeostasis and (ii) to sense mechanical loading and to transmit this information to other cells within the bone to further orchestrate osteoblast and osteoclast function (Burger et al., 1995; Marotti, 2000).
The bone formation activity is consistently coupled to bone resorption that is initiated and maintained by osteoclasts. Osteoclasts are specialized multinucleated cells that originate from the monocyte/macrophage hematopoietic lineage (Fig. 1.4). These cells have the capacity to develop and adhere to bone matrix to then secrete acid and lytic enzymes that degrade and break down the mineral and organic components of bone and calcified cartilage. The matrix degradation process results in the formation of a specialized extracellular compartment known as Howship’s lacuna (Rodan, 1992; Vaananen and Laitala-Leinonen, 2008; Vaananen et al., 2000).