
Pharmaceutical Drug Product Development and Process Optimization
Effective Use of Quality by Design
Sarwar Beg, Majed Al Robaian, Mahfoozur Rahman, Syed Sarim Imam, Nabil Alruwaili, Sunil Kumar Panda, Sarwar Beg, Majed Al Robaian, Mahfoozur Rahman, Syed Sarim Imam, Nabil Alruwaili, Sunil Kumar Panda
- 354 páginas
- English
- ePUB (apto para móviles)
- Disponible en iOS y Android
Pharmaceutical Drug Product Development and Process Optimization
Effective Use of Quality by Design
Sarwar Beg, Majed Al Robaian, Mahfoozur Rahman, Syed Sarim Imam, Nabil Alruwaili, Sunil Kumar Panda, Sarwar Beg, Majed Al Robaian, Mahfoozur Rahman, Syed Sarim Imam, Nabil Alruwaili, Sunil Kumar Panda
Información del libro
Pharmaceutical manufacturers are constantly facing quality crises of drug products, leading to an escalating number of product recalls and rejects. Due to the involvement of multiple factors, the goal of achieving consistent product quality is always a great challenge for pharmaceutical scientists. This volume addresses this challenge by using the Quality by Design (QbD) concept, which was instituted to focus on the systematic development of drug products with predefined objectives to provide enhanced product and process understanding.
This volume presents and discusses the vital precepts underlying the efficient, effective, and cost effective development of pharmaceutical drug products. It focuses on the adoption of systematic quality principles of pharmaceutical development, which is imperative in achieving continuous improvement in end-product quality and also leads to reducing cost, time, and effort, while meeting regulatory requirements. The volume covers the important new advances in the development of solid oral dosage forms, modified release oral dosage forms, parenteral dosage forms, semisolid dosage forms, transdermal drug, delivery systems, inhalational dosage forms, ocular drug delivery systems, nanopharmaceutical products, and nanoparticles for oral delivery.
Preguntas frecuentes
Información
CHAPTER 1
Introduction to Pharmaceutical Product Development
RAHUL SHUKLA*, MAYANK HANDA, and VISHWAS P. PARDHI
1.1 INTRODUCTION
Global Ranking | Company | Revenue in year 2017 (in US billion $)* |
|---|---|---|
1 | Pfizer | 52.54 |
2 | Roche | 44.36 |
3 | Sanofi | 36.66 |
4 | Johnson & Johnson | 36.30 |
5 | Merck | 35.40 |
6 | Novartis | 33.00 |
7 | AbbVie | 28.22 |
8 | Gilead Sciences | 25.65 |
9 | GlaxoSmithKline (GSK) | 24.00 |
10 | Amgen | 22.85 |
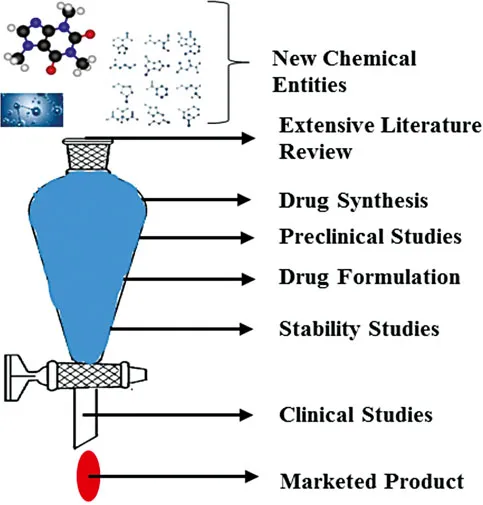
1.2 DRUG DISCOVERY
Global Ranking | Brand | NCEs | Company | Revenue in year 2017 (in US billion $)* |
|---|---|---|---|---|
1 | Humira | Adalimumab | AbbVie Inc. (USA) | 18.43 |
2 | Eylea | Aflibercept | Bayer (Germany) & Regeneron Pharmaceuticals (USA) | 8.23 |
3 | Revlimid | Lenalidomide | Celgene (USA) | 8.19 |
4 | Rituxan | Rituximab | Biogen & Roche | 8.11 |
5 | Enbrel | Etanercept | Amgen Inc. (USA) & Pfizer (Europe) | 7.98 |
6 | Herceptin | Trastuzumab | Roche | 7.55 |
7 | Eliquis | Apixaban | Bristol-Myers Squibb (USA) & Pfizer (USA) | 7.40 |
8 | Avastin | Bevacizumab | Roche | 7.21 |
9 | Remicade | Infliximab | Johnson & Johnson (USA) & Merck (USA) | 7.16 |
10 | Xarelto | Rivaroxaban | Bayer (Germany) & Johnson & Johnson (USA) | 6.54 |
11 | Januvia/Janumet | Sitagliptin | Merck | 5.90 |
12 | Lantus | Insulin glargine | Sanofi | 5.65 |
13 | Prevnar 13/Preventer | Pneumococcal 13-valent conjugate Vaccine | Pfizer | 5.60 |
14 | Opdivo | Nivolumab | Bristol-Myers Squibb | 4.95 |
15 | Neulasta/Peglasta and Neupogen/Gran | Pegfilgrastim and Filgrastim | Amgen (USA) & Kyowa Hakko Kirin (Japan) | 4.56 |
16 | Lyrica | Pregabalin | Pfizer | 4.51 |
17 | Harvoni | Ledipasvir/Sofosbuvir | Gilead Sciences | 4.37 |
18 | Advair/Seretide | Fluticasone and Salmeterol | GlaxoSmithKline | 4.36 |
19 | Tecfidera | Dimethyl fumara... |