
eBook - ePub
Principles of Extractive Metallurgy
F. Habashi
This is a test
Compartir libro
- 494 páginas
- English
- ePUB (apto para móviles)
- Disponible en iOS y Android
eBook - ePub
Principles of Extractive Metallurgy
F. Habashi
Detalles del libro
Vista previa del libro
Índice
Citas
Información del libro
Principles of Extractive Metallurgy was planned in four volumes as follows: Volume 1: General Principles, Volume 2: Hydrometallurgy, Volume 3: Pyrometallurgy, and Volume 4: Electrometallurgy. Volume 1was published in 1969 and is concerned mainly with metallurgical kinetics. Divided into 5 sections, this book explores the scope of pyrometallurgy and pollution problems; the engineering aspects especially on heat transfers; the different processes of preliminary treatment of ores; metal separation by reduction, conversion and other processes; and finally refining processes.
Preguntas frecuentes
¿Cómo cancelo mi suscripción?
¿Cómo descargo los libros?
Por el momento, todos nuestros libros ePub adaptables a dispositivos móviles se pueden descargar a través de la aplicación. La mayor parte de nuestros PDF también se puede descargar y ya estamos trabajando para que el resto también sea descargable. Obtén más información aquí.
¿En qué se diferencian los planes de precios?
Ambos planes te permiten acceder por completo a la biblioteca y a todas las funciones de Perlego. Las únicas diferencias son el precio y el período de suscripción: con el plan anual ahorrarás en torno a un 30 % en comparación con 12 meses de un plan mensual.
¿Qué es Perlego?
Somos un servicio de suscripción de libros de texto en línea que te permite acceder a toda una biblioteca en línea por menos de lo que cuesta un libro al mes. Con más de un millón de libros sobre más de 1000 categorías, ¡tenemos todo lo que necesitas! Obtén más información aquí.
¿Perlego ofrece la función de texto a voz?
Busca el símbolo de lectura en voz alta en tu próximo libro para ver si puedes escucharlo. La herramienta de lectura en voz alta lee el texto en voz alta por ti, resaltando el texto a medida que se lee. Puedes pausarla, acelerarla y ralentizarla. Obtén más información aquí.
¿Es Principles of Extractive Metallurgy un PDF/ePUB en línea?
Sí, puedes acceder a Principles of Extractive Metallurgy de F. Habashi en formato PDF o ePUB, así como a otros libros populares de Technology & Engineering y Materials Science. Tenemos más de un millón de libros disponibles en nuestro catálogo para que explores.
Información
PART ONE
General
CHAPTER ONE
Scope
INTRODUCTION
Formation of immiscible molten layers
Slags
ENGINEERING ASPECTS
Heat transfer
Compaction of powders
Solid-gas separation
Oxidation of a solid phase
Oxidation in molten phase
Metallothermic reactions
CHEMICAL ASPECTS
Preliminary treatment of ores
Meta1 separation
Refining
INTRODUCTION
PYROMETALLURGY IS that branch of extractive metallurgy which deals with the extraction of metals from their ores by thermal methods. Because of the extensive use of carbon as a fuel and as a reducing agent, pyrometallurgy can be called the technology of carbon, in analogy to organic chemistry, the chemistry of carbon. More than 50% of the coal mined today is used by the metallurgical industry. Pyrometallurgy is the most important division of extractive metallurgy, since it is involved in the recovery of most metals. It is conveniently studied from two points of view:
(1) The chemical aspects and
(2) The engineering aspects.
Before discussing these aspects, it would be advantageous first to point out an important phenomenon in pyrometallurgy, namely, the formation of immiscible molten layers.
Formation of immiscible molten layers
During the melting of an ore or a concentrate, the silicates, sulfides, arsenides, and metals produced coalesce, each forming an individual molten layer. Because of the differences in specific gravity, it is usually possible to allow the material to settle and to separate each layer. The silicate layer on the top is called slag, the sulfide layer next is called matte, and the arsenide layer next, is called speiss. Table 1-1 gives the properties of the different layers formed when melting lead oxide (obtained by the oxidation of a lead sulfide concentrate) in the presence of carbon to get impure metallic lead called bullion. The separation is, however, not sharp; each layer will contain some impurities from the other layers in contact with it. Not all four layers are necessily formed in a process. Most common are the slag-matte or slag-metal layer that form. The case of lead is a particular example.
Table 1-1. Immiscible molten layers formed during the production of lead
Hot gases leaving the furnace are loaded with dust particles. These are mainly components of the charge as well as volatile components formed during melting, e.g., cadmium and indium. In a pyrometallurgical plant, provision is always made to separate the dust from the gas for recovery and to avoid pollution of the environment.
To facilitate the melting process, a flux is usually added to combine with the high-melting point components and form the slag having a low melting point. For example, an ore containing limestone as gangue mineral when heated will result in the formation of CaO which has a melting point of 2580°C. To melt such material, a large amount of fuel will be consumed to achieve this temperature. This approach is not only uneconomical but also will necessitate the construction of a furnace that can withstand such high temperatures. If, however, a flux such as silica (melting point 1728°C) is added to the ore, then on heating, a reaction between CaO and SiO2 will take place to form calcium silicate, i.e., a slag whose melting point may be as low as 1500°C. In this way the gangue minerals are separated from the other valuable ore components in the form of a low-melting slag.
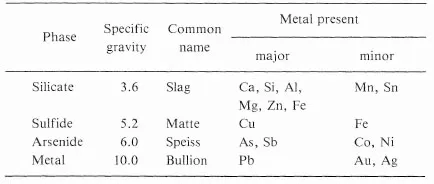
If on the other hand, the gangue mineral was SiO2, then CaO should be added as a flux to aid in the formation of the slag. Fig. 1-1 shows the phase diagram SiO2—CaO.
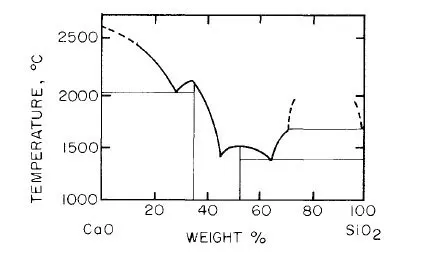
Fig. 1-1. Simplified phase diagram of SiO2—CaO system.
Slags
In pyrometallurgy, SiO2 is considered an acidic oxide and CaO as basic oxide. This concept was introduced long ago when it was observed that some oxides dissolve in water forming an acid, and others when dissolved form a base*. Although SiO2 is insoluble in water, it was regarded as the anhydride of various silicic acids: ortho H4SiO4, meta H2SiO3, and poly H4SiO8, in which the hydrogen atoms can be replaced by metals to form silicates. This is true for a limited number of silicates such as those of the alkali metal group. Recent research, however, has shown that the structure of other silicates is not so simple. Thus, it was found that SiO2 is composed of a large network in which the fundamental structural units is the tetrachedral group SiO4; the central silicon ion is linked with four oxygen ions as shown in Fig. 1-2 and 1-3. In the molten state the network is slightly distored.
In terms of recent theories, and oxide, MO is called a basic oxide because it provides oxygen ions
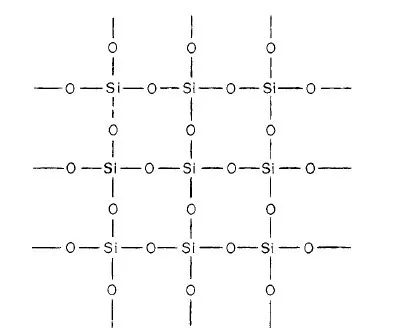
Fig. 1-2. Two-dimensional representation of the structure of SiO2.
Fig. 1-3. Three dimensional representation of the structure of SiO2.
● Si4+
○ O2−
An acidic oxide, on the other hand, will absorb ions provided by a basic oxide, e.g.:
Figs. 1-4 and 1-5 represent the reaction of CaO with SiO2 to form calcium silicate slag. It can be seen that when CaO dissolves in molten silica, the SiO bonds are steadily broken down, until eventually the melt is composed of discrete groups and Ca2+ ions. A slag, therefore, is a silicate network of various composition. It is considered an acid slag when it is rich in silica, and basic slag when poor in silica, i.e., rich in basic oxides. An acid slag will be capable of dissolving basic oxides while a basic slag, on the other hand, will be capable of dissolving acidic oxides.
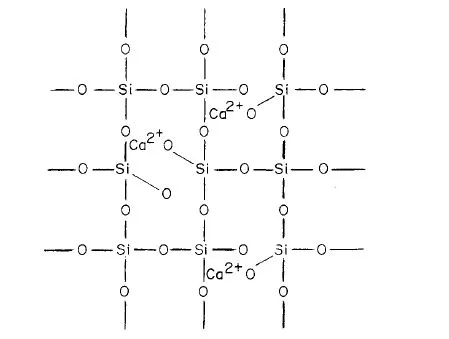
Fig. 1-4. TWo dimensional representation of cal...