
eBook - ePub
Microfiltration and Ultrafiltration
Principles and Applications
Leos J. Zeman, Andrew L. Zydney
This is a test
Compartir libro
- 642 páginas
- English
- ePUB (apto para móviles)
- Disponible en iOS y Android
eBook - ePub
Microfiltration and Ultrafiltration
Principles and Applications
Leos J. Zeman, Andrew L. Zydney
Detalles del libro
Vista previa del libro
Índice
Citas
Información del libro
Integrates knowledge on microfiltration and ultrification, membrane chemistry, and characterization methods with the engineering and economic aspects of device performance, device and module design, processes, and applications. The text provides a discussion of membrane fundamentals and an analytical framework for designing and developing new filtrations systems for a broad range of technologically important functions. It offers information on membrane liquid precursors, fractal and stochastic pore space analysis, novel and advanced module designs, and original process design calculations.
Preguntas frecuentes
¿Cómo cancelo mi suscripción?
¿Cómo descargo los libros?
Por el momento, todos nuestros libros ePub adaptables a dispositivos móviles se pueden descargar a través de la aplicación. La mayor parte de nuestros PDF también se puede descargar y ya estamos trabajando para que el resto también sea descargable. Obtén más información aquí.
¿En qué se diferencian los planes de precios?
Ambos planes te permiten acceder por completo a la biblioteca y a todas las funciones de Perlego. Las únicas diferencias son el precio y el período de suscripción: con el plan anual ahorrarás en torno a un 30 % en comparación con 12 meses de un plan mensual.
¿Qué es Perlego?
Somos un servicio de suscripción de libros de texto en línea que te permite acceder a toda una biblioteca en línea por menos de lo que cuesta un libro al mes. Con más de un millón de libros sobre más de 1000 categorías, ¡tenemos todo lo que necesitas! Obtén más información aquí.
¿Perlego ofrece la función de texto a voz?
Busca el símbolo de lectura en voz alta en tu próximo libro para ver si puedes escucharlo. La herramienta de lectura en voz alta lee el texto en voz alta por ti, resaltando el texto a medida que se lee. Puedes pausarla, acelerarla y ralentizarla. Obtén más información aquí.
¿Es Microfiltration and Ultrafiltration un PDF/ePUB en línea?
Sí, puedes acceder a Microfiltration and Ultrafiltration de Leos J. Zeman, Andrew L. Zydney en formato PDF o ePUB, así como a otros libros populares de Medizin y Biochemie in der Medizin. Tenemos más de un millón de libros disponibles en nuestro catálogo para que explores.
Información
I
SCIENTIFIC AND MANUFACTURING ASPECTS OF MF/UF MEMBRANES
Seek simplicity, and distrust it.
—Alfred North Whitehead English mathematician and philosopher, 1861-1947
1
Basic Chemistry and Physics of MF/ UF Membranes and Their Precursors
I Membrane Surface Chemistry
In the context of this book, a membrane refers to a synthetic (most often polymeric) selective barrier, used in industrial or lab-scale processes of microfiltration (MF) or ultrafiltration (UF). In those processes, certain feed stream components are permitted passage by the membrane (more strictly, by its pores) into a permeate stream, while other, usually larger feed components, are retained (rejected) by the membrane. These rejected species accumulate in the retentate stream. Retention and rejection are regarded as synonymous terms in this book. As explained in Section II, the distinction between MF and UF lies primarily in the size distribution of the membrane pores.
By membrane chemistry we usually mean the chemical nature and composition of the membrane surface. We are concerned here particularly with that part of the surface that is in contact with the processed stream. The chemical makeup of the surface may often be quite different from that found in the membrane bulk. Such differences may be caused by material partitioning during membrane formation (more on this in Chapter 2), or by some selected surface postformation treatments. Membrane chemistry determines such important properties as hydrophilicity or hy-drophobicity, presence or absence of ionic charges, chemical and thermal resistance, binding affinity for solutes or particles, biocompatibility, etc.
The MF/UF membrane chemistry can be modified to improve performance in targeted applications. Almost 50% of all MF/UF membranes marketed today are surface-modified. The common membrane modification strategies involve: (1) addition of a compatible modifier (such as a hydrophilic or charged polymer) into the casting solution (lacquer); (2) adsorption of a modifier onto the membrane surface ; (3) chemical or physicochemical post-treatments of the surface (e.g., hydrolysis or gas plasma treatment); and/or (4) grafting or cross-linking a modifier on the surface. Postformation modifications (2-4) are sometimes performed by practitioners without access to the membrane formation technology and equipment. Numerous other special methods of membrane chemistry modification have been developed. A distinct case of surface modification is the attachment of ligands, enzymes, or catalytic groups to the surface of the membrane. Such type of modification is used to form affinity or membrane reactor membranes. However, our emphasis in this book will be primarily on membranes used in the “classical” filtration applications within the field of MF and UF separations.
Hydrophilicity of surfaces is expressed most conveniently in terms of the water contact angle, θ. Hydrophilic surfaces have the contact angle, θ, close to 0° [i.e., cos(θ) = 1], while more hydrophobic materials exhibit the contact angle close to or above 90° [i.e. cos(θ) ≤ 0]. The stationary interfacial balance of forces that determines the value of the contact angle is shown (two-dimensionally) in Fig. 1. At equilibrium, three surface (interfacial) tensions, γ, corresponding to solid/vapor (sv), solid/liquid (si), and liquid/vapor (lv) interfaces, are counterbalanced. Water molecules are present not only in the liquid drop but also in the vapor, and on the surface of the solid outside of the drop. The equation shown in Fig. 1 is referred to as Young’s or the Young-Dupré equation. To this author’s knowledge, it has never been verified experimentally due to the obvious difficulty of measuring the sv interfacial tension.
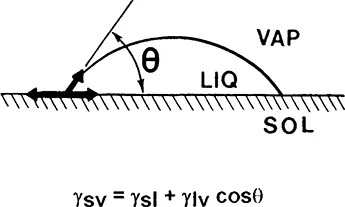
Figure 1 Balance of interfacial tensions, γi, for a sessile drop of liquid (I) (water) on a flat solid (s) surface in the presence of vapor (v). The equilibrium contact angle, θ, is related to the three interfacial tensions according to the Young’s equation.
The consequence of the contact angle phenomenon for the capillary (or pore) intrusion behavior (which is important for membranes) is shown schematically in Fig. 2. A difference is shown here between spontaneous water wetting (wicking) in a “hydrophilic” pore, and nonwetting in a “hydrophobic” pore. It is important to mention that many “hydrophobic” materials do actually wet as long as the water/material contact angle is below 90°. In practice, however, their wetting may be slow or incomplete, and low surface tension liquids (e.g., common alcohols or surfactant solutions) are used to enhance wetting. Application of a sufficiently high pressure can also produce intrusion (see Chapter 4, Section I).
A complication appears in this simple picture. In any dynamic (moving boundary) measurement, two contact angles are usually obtained. They are referred to as the receding and advancing contact angles, and the divergent behavior is called the contact angle hysteresis. Receding contact angles are usually lower than their advancing counterparts. More than one reason may exist for such hysteresis behavior: surface contamination, surface roughness, solid porosity, heterogeneity, etc. However, with polymer solids, the most likely cause is the polymer surface molecular rearrangement (conditioning) at the si interface that leads to the lowering of the receding angle value.
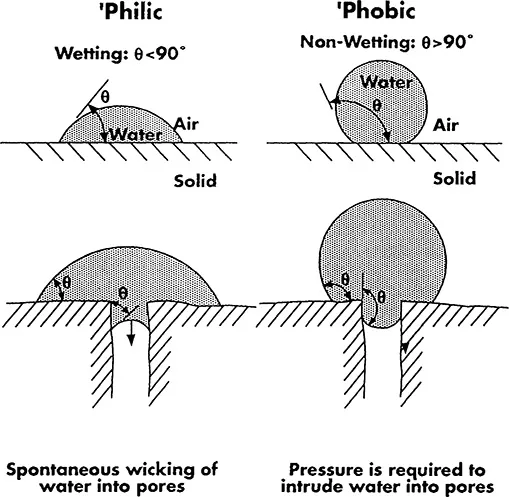
Figure 2 The effect of the equilibrium contact angle, θ, on the pore intrusion phenomenon. Water intrudes spontaneously (wicks) Into pores if θ < 90° (a hydrophilic solid case). No intrusion occurs (in the absence of an applied pressure) if θ > 90° (a hydrophobic solid case).
Figure 3 shows the experimental determination (in the author’s laboratory) of the two dynamic water contact angles on a nonporous poly-vinylfluoride (Tediar polyvinyl fluoride) film. The experimental technique used was the so-called Wilhe...