![]()
Chapter 1 | Principles and applications of bioprinting A. Skardal |
Contents
1.1 Introduction
1.2 Bioprinting—then and now
1.2.1 Pioneers
1.2.2 Modalities defined
1.2.2.1 Inkjet
1.2.2.2 Extrusion
1.2.2.3 Stereolithography and projection patterning
1.2.2.4 Laser-induced forward transfer
1.2.3 Wider adoption
1.3 Essential components of bioprinting
1.3.1 Cells
1.3.1.1 Cell lines
1.3.1.2 Primary cells
1.3.1.3 Stem cells and stem cell-derived cells
1.3.2 Biomaterials
1.4 Future hurdles and potential
1.4.1 Bioink limitations
1.4.2 Resolution versus speed
1.4.3 Regulatory hurdles
1.5 Conclusion
References
1.1 Introduction
Bioprinting has emerged as a flexible tool in regenerative medicine with potential in a variety of applications. Bioprinting is a relatively new field within biotechnology that can be described as robotic additive biofabrication that has the potential to build or pattern viable organ-like or tissue structures in three dimensions.1 In general, bioprinting uses a computer-controlled three-dimensional (3D) printing device to accurately deposit cells and biomaterials into precise geometries with the goal being the creation of anatomically correct biological structures. Generally, bioprinting devices have the ability to print cell aggregates, cells encapsulated in hydrogels or viscous fluids, or cell-seeded microcarriers—all of which can be referred to as bioink—as well as cell-free polymers that provide mechanical structure or act as placeholders.2,3 Biologically inspired, physiologically relevant computer-assisted designs can be used to design and guide the placement of specific types of cells and materials into precise, planned geometries that mimic the architecture of actual tissue construction,4 which can subsequently be matured into functional tissue constructs or organs.5,6
There is already a massive shortage of donor organs for implantation in patients.7,8 For example, as of July 2017, over 117,000 patients were still on waiting lists for donor organs, and there are only 8,096 currently identified available donors.9 Today, most transplants have high success rates. Kidney, pancreas, liver, intestine, and heart transplants have more than 80% 1-year survival rates and more than 70% 5-year survival rate (with the exception of intestine).10 These statistics illustrate the demand for an increased organ supply, as well as the fact that implanting organs is a useful and effective therapy. Furthermore, while preclinical testing of candidate drugs in animals is well established, it is neither particularly efficient nor is it always predictive of the clinical outcome in humans.11 3D human tissues, not animal tissues, are logically the most appropriate models for screening drugs meant for humans and investigating diseases afflicting humans. Bioprinted tissues and organs have the potential to address the need for implantable tissues for patients waiting on donor lists and testable human tissues for research and development.
1.2 Bioprinting—then and now
Bioprinting is a young scientific undertaking when compared with many other areas of research and technological development. Yet now, it has been around for nearly a decade and a half. In that time, the field has made extraordinary advances in some respects, but remains quite stagnant in others. As such, it makes sense for those of us who toil on this exciting field to compare where we are now to where we were when bioprinting was in infancy, thereby identifying the limitations and technical challenges that remain to be overcome before bioprinting can be fully scaled to biomanufacturing levels.
1.2.1 Pioneers
3D bioprinting arose from the multidisciplinary amalgamation of several other relatively cutting-edge technologies—additive manufacturing and cell patterning. Additive manufacturing had been present for some time for other applications, fabricating devices, components, and parts from materials such as metals and plastics. Cell patterning and substrate patterning were related technologies employed in research labs for applications such as probing cell–protein interactions. In the early 2000s, several researchers: Vladimir Mironov, Gabor Forgacs, and Thomas Boland, saw the natural combining of these technologies, and others, such as commercial inkjet printing, as a way to build 3D living structures that perhaps one day could serve as replacement tissues and organs in human patients.3,5,12 Thus was coined the term organ printing, the precursor to bioprinting, which is the term we have more broadly settled on.
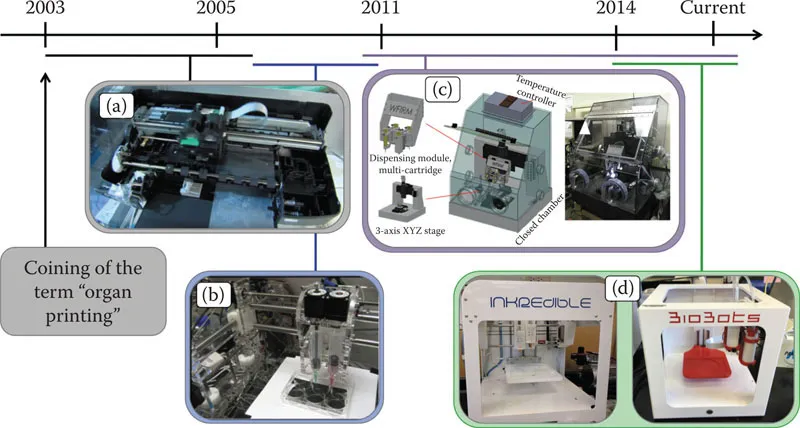
Bioprinting hardware has evolved significantly (Figure 1.1). Early bioprinters were often custom built, hacked, and inkjet printers.13,14 The few labs working in these areas built their hardware themselves, and these custom devices were often incredibly difficult to operate, full of software bugs, and featured impossible user interfaces. Those lucky enough to receive substantial funding could utilize other 3D-printing devices that were commercially available, but these devices were not engineered to print biological materials, and ran U.S.$100,000 to U.S.$200,000 for a single operational piece of hardware.15 During this time, additive manufacturing continued to advance, particularly in the open-source world, resulting in a number of inexpensive, but still buggy, printers that were amenable to bioprinting, but only after substantial tinkering. These limitations made advancing of bioprinting technology difficult, but not impossible.
Figure 1.1 Evolution and examples of bioprinting hardware platforms. (a) A modified HP commercial inkjet printer for printing cells. (b) An open-source, Fab@Home “DIY” 3D printer employed for bioprinting purposes. (c) A custom-built 3D bioprinter with multinozzle capacity for printing hydrogels and melt-cure polymers. (d) Examples of relatively recent, commercially available bioprinters that are affordable for most biomedical research laboratories.
During this time, the technology continued to evolve and bifurcate, as did the terminology associated with other facets of bioprinting. Boland-led efforts continued to push inkjet-based bioprinting. In parallel, the Forgacs-led Frontiers in Integrated Biological Research (FIBR) effort built on biophysics concepts, that is, tissue liquidity and tissue fusion, developing a platform in which cell aggregates, or tissue spheroids, were deposited into a hydrogel biomaterial substrate, and based on both cell–cell and cell–matrix-based interactions would fuse in a controllable manner into larger bioengineered tissue constructs.14,16,17,18,19,20,21 Within this paradigm was coined the term bioink, referring to the cell aggregates. Likewise, the term biopaper was coined to reflect the hydrogel biomaterial component. Although over time, the term biopaper has all but disappeared as in the minds of most researchers, bioink encompasses cells, biomaterials, and combinations thereof.
1.2.2 Modalities defined
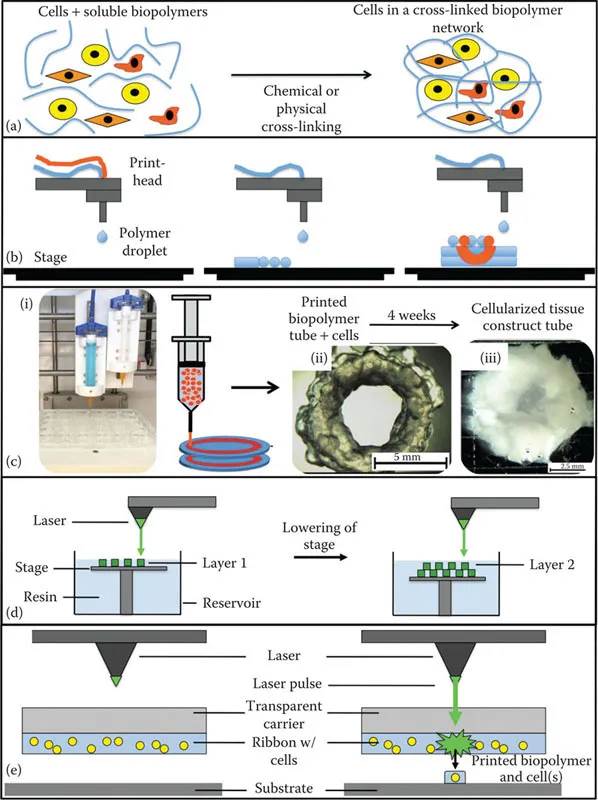
During this formative stage, the continued bifurcation of approaches occurred. The spheroid-in-hydrogel methodology evolved into two independent modalities. Cell aggregate bioprinting, and later cell–rod or cell–filament bioprinting techniques, became referred to as scaffold-free bioprinting, as this approach no longer relied on a biologically supportive hydrogel environment. Rather, inert materials such as agarose were used to physically support 3D arrays of cell aggregates and filaments while they formed into more complex structures through tissue fusion after which the agarose supports were removed. During the same period the materials that were developed as the biopaper to the cellular bioink were explored more directly as a bioink of sorts themselves, containing cells encapsulated within (Figure 1.2a). This approach, in which cells are generally encapsulated in a material, usually a hydrogel, and then printed from a syringe tip-like print head, is what is now referred to as extrusion bioprinting, and a number of labs and companies have their own bioink formulations.
1.2.2.1 Inkjet
The first printing modality that we describe is inkjet bioprinting (Figure 1.2b), which can be further broken down into two approaches: (1) thermal and (2) piezoelectric. These two approaches have a similar overall methodology in which a printing cartridge or syringe is filled with bioink that is then forced through an output aperture. Thermal inkjet bioprinters are originally derived from the technology used in commercially available desktop inkjet printers, as we have described in the evolution of bioprinting earlier. In early instances of this approach, commercially available ink cartridges served as the print heads. The ink from these cartridges were removed and replaced with a bioink.22,23 Thermal inkjets then use a heating element that creates a bubble in the bioink, generating pressure that forces the bioink through the output aperture of the print head. The specific parameters of these print heads are very much dependent on the particular type of cartridge used. Spatial resolution, in particular, is dependent on the type of inkjet printer that is being used, the size of the output aperture, and burst level and temperature driven by the thermal unit. In addition, spatial precision can be adjusted by altering the composition and properties of the bioink, such as concentration and viscosity, in relation to the average drop volume of the device.
Figure 1.2 Bioprinting hardware modalities. (a) Prior and/or during bioprinting, cells are encapsulated within bioink biomaterials. Schematics describing (b) inkjet bioprinting, (c) extrusion bioprinting (i - an extrusion bioprinter; ii - a tubular construct immediately after printing; iii - over time the tubular construct is remodeled by the cells within into living tissue.), (d) stereolithography, and (e) laser-induced forward transfer (LIFT) bioprinting.
Throughput in inkjet bioprinters can vary widely, and these printers with small output apertures have a tendency to rapidly clog if printing parameters or bioink properties are not optimal. Some groups have experimented with adding chemical agents to bioink formulations in an effort to alleviate clogging,24 but this needs careful consideration to ensure that these supplements do not cause cell toxicity. Low overall throughput is another concern that prevents thermal inkjet printing from being used more widely for bioprinting applications. Small droplet volumes, and a lack continues deposition, results in incr...