
eBook - ePub
The Science For Conservators Series
Volume 3: Adhesives and Coatings
Conservation Unit Museums and Galleries Commission
This is a test
Compartir libro
- 140 páginas
- English
- ePUB (apto para móviles)
- Disponible en iOS y Android
eBook - ePub
The Science For Conservators Series
Volume 3: Adhesives and Coatings
Conservation Unit Museums and Galleries Commission
Detalles del libro
Vista previa del libro
Índice
Citas
Información del libro
For more than ten years, the Science for Conservators Series have been the key basic texts for conservators throughout the world. Scientific concepts are basic ot the conservation of artefacts of every type, yet many conservators have little or no scientific training. These introductory volumes provide non-scientists with the essential theoretical background to their work.
Preguntas frecuentes
¿Cómo cancelo mi suscripción?
¿Cómo descargo los libros?
Por el momento, todos nuestros libros ePub adaptables a dispositivos móviles se pueden descargar a través de la aplicación. La mayor parte de nuestros PDF también se puede descargar y ya estamos trabajando para que el resto también sea descargable. Obtén más información aquí.
¿En qué se diferencian los planes de precios?
Ambos planes te permiten acceder por completo a la biblioteca y a todas las funciones de Perlego. Las únicas diferencias son el precio y el período de suscripción: con el plan anual ahorrarás en torno a un 30 % en comparación con 12 meses de un plan mensual.
¿Qué es Perlego?
Somos un servicio de suscripción de libros de texto en línea que te permite acceder a toda una biblioteca en línea por menos de lo que cuesta un libro al mes. Con más de un millón de libros sobre más de 1000 categorías, ¡tenemos todo lo que necesitas! Obtén más información aquí.
¿Perlego ofrece la función de texto a voz?
Busca el símbolo de lectura en voz alta en tu próximo libro para ver si puedes escucharlo. La herramienta de lectura en voz alta lee el texto en voz alta por ti, resaltando el texto a medida que se lee. Puedes pausarla, acelerarla y ralentizarla. Obtén más información aquí.
¿Es The Science For Conservators Series un PDF/ePUB en línea?
Sí, puedes acceder a The Science For Conservators Series de Conservation Unit Museums and Galleries Commission en formato PDF o ePUB, así como a otros libros populares de Business y Amministrazione museale. Tenemos más de un millón de libros disponibles en nuestro catálogo para que explores.
Información
1
Sticking things together
A How do things stick together?
B What is needed to make a good joint?
C Helping the adhesive to do its job
Sticking things together
In this chapter the question “What makes things stick together?” is tackled. The requirements of a good adhesive and a good joint are also considered, and, finally, the characteristics, advantages and disadvantages of the various types of adhesives used in conservation.
A How do things stick together?
The objects you deal with are integral entities, that is, they stay in one piece, because they are held together by the strong cohesive forces that stem from either ionic, covalent or metallic primary bonds between their constituent atoms (Book 1, Chapter 4), or from secondary bonds between their molecules (Book 2, Chapter 1). As you know from common experience of a broken object, a cup say, even if you fit the pieces back together very carefully they will not stick, and you can still see the line of the break. Although the thickness of this line can be extremely small — a fraction of a millimetre — it is still very large compared with the distance over which the cohesive forces act.
One reason why the broken surfaces cannot be put back into intimate contact is that on a microscopic scale they are, in fact, very irregular. Even the fracture surface of a piece of glass, which we see with the naked eye as being extremely smooth, is revealed by a powerful microscope to be a mazy scene of ridges and valleys (see Figure 1.1). It is not surprising therefore that two such surfaces cannot be perfectly matched again: they will only make contact on a microscopic scale at rare points, and over most of the surface there will be air between the pieces.

Figure 1.1 The fracture surface of a piece of glass as seen through a powerful microscope, showing ridges and valleys.
Further, immediately an object is broken, the newly created fracture surfaces become contaminated by oxygen, water or other chemicals in the environment. Those atoms or molecules at the surface of the object that, prior to fracture, contributed to the cohesive forces in the material are now free to make bonds with the contaminant atoms or molecules. In this way metals oxidise by primary bonds being formed between metal and oxygen atoms; water molecules from the atmosphere hydrogen-bond to materials such as paper and wood leading to a thin layer of moisture on the surface, and so on. Processes like these are the ones that have to be reversed when an object is cleaned. Surface contaminants adhere,via primary or secondary bonds, to a fracture surface, and so they inhibit the rejoining of the broken pieces.
Since the broken surfaces will not join themselves together, another way must be found. Basically, there are two ways by which bits of solid materials can be joined together:
1 Using some sort of device that mechanically locks the pieces together. (Mechanical in this context means non-chemical.)
2 Using an adhesive; that is, a material which, ideally, fills the gaps between the pieces, adheres to both surfaces and achieves a sufficiently strong and rigid interface between the pieces.
Sewing torn pieces of fabric together is an obvious example of mechanical locking, and so is the use of screws, rivets and dowels.
The joining mechanism does not depend on any primary or secon dary bonding between the locking device and the pieces of the object. Of course, chemical reactions may occur with time; the rusting of screws, and the rotting of threads are examples which may lead to breakdown of the joint.
Adhesives have been the subject of considerable scientific research, but exactly how they bond to solids is still not clearly understood. It is thought that adhesion is due to secondary bonding between the molecules in the adhesive and the atoms or molecules at the surface of the pieces to be joined (the adherend or substrate).Clearly, the magnitude of these forces is an important factor in determining the strength of the resulting joint. Nowadays, following the development of a wide variety of synthetic polymers, a multitude of new adhesives of this type is available in addition to the traditional natural polymeric adhesives made from animal hide and bone, starch, cellulose, and so on.
Some adhesives are formed by chemical reactions in situ between different ingredients. This happens, for instance, with epoxy resins and cyanoacrylate adhesives. Of course, in a chemical reaction, primary bonds in the starting materials are broken and new ones are made to form the products of the reaction. Even with these adhesives it is thought that no new primary bonds are formed across the interface between the adhesive and the solid surface, but that adhesion is essentially by means of secondary bonds.
In some particular cases the joining process does produce primary bonding right across the interface, so much so that the original interface disappears. This happens, for instance, when a joint is made by locally melting the two pieces and holding them together until they resolidify; examples are brazing and welding. Figure 1.2 is an illustration of how the interface is destroyed by welding. From
Figure 1.2 A photomicrograph of a welded joint, showing how an interface between two pieces of metal has been destroyed by fusion.
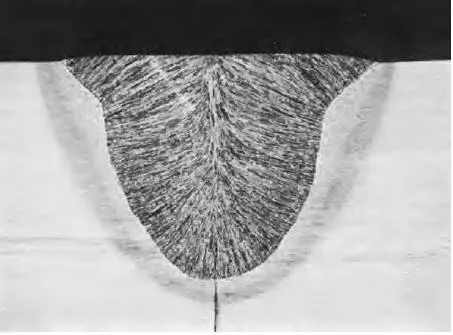
a conservation viewpoint, such a technique has some obvious undesirable features.
The difference between the two ways of joining — by mechanical locking or by an adhesive — becomes rather blurred when you consider how an adhesive works at a microscopic level. In fact, in many cases, mechanical interlocking may contribute significantly to the effectiveness of an adhesive. If you look again at the glass fracture surface illustrated in Figure 1.1, you will appreciate that a suitable adhesive could penetrate the nooks and crannies on the surface, and also the surface to which it is to be joined, and thus produce an interlocking of the fragments. Similarly, pieces of paper or fabric can be joined by an adhesive that penetrates between the fibres. The pieces are held together by the veins of adhesive, which act as hooks around the paper or textile fibres, as in Velcro.
In the context of this book there is no need to pursue the details of theories of adhesion any further, however the basic mechanisms of secondary bonding and microscopic interlocking just described will enlighten later discussions. In the next section the characteristics that an adhesive and the surfaces to be joined should have in order to produce a satisfactory joint are explored.
B What is needed to make a good joint?
What is meant by a good joint really depends on the meaning of “good”. It involves a balance between judgements about the quality of the joint on technical, aesthetic and ethical grounds, the required properties of the adhesive, and the efficiency with which the joint is made.
At this point it is important to appreciate that in discussing different types of adhesive, and the science relating to them, there is a very particular definition of “good” when considering adhesives for conservation work. Most modern adhesives have been developed to produce joints that are good in terms of the criteria of mass-production manufacturing industries — furniture production, car production and so on. This has meant that the search has been for adhesives that produce very strong joints — indeed with many modern adhesives the material of which the object is made may break, rather than the joint. Further, the requirement is for joints which resist degradation in use caused by chemicals in the environment. This usually means that joints produced by such adhesives are difficult to reverse by, for example, dissolving in common solvents such as water and simple organic liquids. These needs for strong, chemically indestructible adhesives are usually at odds with those of the conservator, who often wishes to make a glued joint which holds the pieces of an object together satisfactorily but which can be readily taken apart at some future time without damaging its characteristics.
Although these very different requirements are quite obvious, ways of considering the scientific background to adhesives in the context of conservation are not. For instance, it is more straightforward to analyse the factors that produce a strong joint rather than those that produce a weak joint. This is the approach taken here, with, where appropriate, a discussion of the special requirements of adhesives in conservation work.
Think of an object that needs to be mended. What are the important requirements of the joint you intend to make? What are the steps in making the joint that need the most careful attention?
The diagram in Figure 1.3 attempts to cover the main points you should have considered. Notice that many of the factors have implications for others. For example, making a joint strong may not be compatible with the necessity to remove (take down) the joint without harming the object, and an invisible join may soon become visible due to discoloration. All of the topics in Figure 1.3 (except the need to have clean surfaces) have implications for the type of adhesive you use; they can be used to draw up a specification of the desirable properties of an adhesive. However, there may be additional constraints put on the choice of adhesive, such as cost and whether it presents hazards to the conservator, for example by releasing noxious fumes.
...