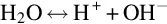The chemical, physical and biological characteristics of water combine to form what is called water quality. Even minute changes in these characteristics can jeopardize the lives and industries that depend on water. To preserve its quality, accurate monitoring of water parameters such as conductivity, pH, salinity, temperature, dissolved oxygen (DO), and turbidity is crucial. This chapter describes a broad selection of water quality sensors that are suitable for both simple spot sampling requirements and complex unattended monitoring projects.
1.1 pH Measurement and Value
1.1.1 pH and How to Measure It
Knowing the pH value of a solution or fluid is fundamental in many chemical and analytical tasks and its measurement often determines any follow-up measurements. Measuring pH can seem to be trivial, which is why pH measurements are often not questioned. But to make a useful pH measurement, close attention must be paid to the measurement details. To determine pH accurately and avoid errors, you must first be familiar with the basics of pH measurement.
1.1.2 What Does the pH Value of a pH Measurement Mean?
The water molecule has the property of dissociating into two ionic components in aqueous solutions (Covington et al., 1985).
The H+ ion is termed a hydrogen ion or proton, and the OH– ion is the hydroxide ion. The pH value describes the activity of hydrogen ions in aqueous solutions, typically on a scale of 0–14. Based on this pH scale, liquids are characterized as being acidic, alkaline or neutral; a solution which is neither acidic nor alkaline is neutral, which corresponds to a value of 7 on the pH scale. Acidity indicates a higher activity of hydrogen ions and a pH measurement value lower than 7. Alkaline solutions are characterized by a lower hydrogen ion activity or higher hydroxide ion activity, respectively, and a pH measurement value above 7.
The pH scale is logarithmic. A difference of one pH measurement unit represents a 10-fold, or 10 times, increase or reduction of hydrogen ion activity in the solution. This explains how a solution's aggressiveness increases with the distance from the neutral point.
One of the keys to understanding pH measurements is the term “activity”; because the activity is temperature dependent, it is not the same as the solution's concentration. Activity, a, is defined as the product of the activity coefficient y, which is always smaller than 1, and the actual concentration c of the concerned compound (a = y × c).
Activity is the effective concentration of a chemical compound, or more precisely its particles in the solution. In a real solution the activity is always smaller than the actual concentration. This is true because it is only in an ideal (infinitely thinned) solution that the soluted particles do not affect each other. In this case they are spread apart, because many molecules of the solvent are between them. The difference between activity and concentration becomes apparent in real solutions of ions, because ions interact with each other as a result of their electrical charge. To describe or calculate the characteristics of a solution as exactly as possible, the activity and not the concentration must be used in the mass action law.
1.1.3 How Do I Measure the pH Value?
The pH value can be measured using electrochemical measuring systems, litmus paper, or indicators and colorimeters. The easiest way to take a pH measurement is to use litmus paper or a colorimeter. The advantage of this type of pH measurement is that the pH range is well known and the methods are easy to apply. Unfortunately, in many cases litmus paper and colorimeters are not accurate enough to make high-quality pH measurements, because the pH value transition point depends on the user.
Another pH value measurement possibility is amperometry (Stredansky et al., 2000). The advantage of amperometry as a pH measurement method is that it is simple to use. In amperometric pH measurements hydrogen generation occurs on a noble metal. When combined with a less noble metal, a power distributing galvanic cell is formed. Because hydrogen ions are generated, the cell's current depends on the pH value. The disadvantages of this method are that differences in the sample composition create very large errors in pH measurements and the method cannot deliver dependable results in extremely concentrated acids and bases, due to effects related to the pH glass membrane.
In special cases, the pH value measurement can be made using conductometry (Jacobs et al., 1992). With this method any membrane effects are minimized because of the measurement technique. The advantage of this pH measurement method is that it is relatively easy to use. The disadvantage is that a conductivity measurement measures all ion activity, not just hydrogen ion activity. Additionally, this pH measurement is only reproducible and safe at low ion concentrations.
A relatively new method for pH value measurement is the ion selective field effect transistor (ISFET) (Duroux et al., 1991). Briefly, the ISFET is a transistor with power source and drain, divided by an isolator. This isolator (gate) is made of a metal oxide where hydrogen ions accumulate in the same way as an electrode. The positive charge that accumulates outside the gate is “mirrored” inside the gate by an equal negative charge generated. Once this happens, the gate begins to conduct electricity. The lower the pH value, the more hydrogen ions accumulate and the more current can flow between source and drain. The ISFET sensors, similar to glass pH electrodes, act according to the Nernst equation. The advantage of an ISFET is that it is very small. The actual field effect transistor (FET) is only 0.2 mm2. The disadvantage of using an ISFET for pH measurements is that they have comparatively short durability and low long-term stability, with a typical use life cycle being in the range of weeks.
The most common method of pH value measurement is the use of pH measurement electrodes, like the IoLine series from SI Analytics. These pH measurement devices are electrochemical sensors that consist of a measuring electrode and a reference electrode. The pH measurement electrode is made of special glass which, due to its surface properties, is particularly sensitive to hydrogen ions. The pH measurement electrode is filled with a buffer solution with a pH val...