![]()
Introduction to Part 1
Introduction to Part 1
1 Chemiosmotic Energy Transduction
2 Ion Transport Across Energy-Conserving Membranes
3 Quantitative Bioenergetics
4 The Chemiosmotic Proton Circuit in Isolated Organelles
![]()
Introduction to Part 1
Because all biochemical reactions involve energy changes, the term bioenergetics could validly be applied to the whole of life sciences. However, in the field of biochemistry and biology, the term originally came to mean the study of the energy conversion processes that occur on, in, or across the inner mitochondrial membrane, the cytoplasmic membranes of bacteria and the photosynthetic thylakoid membranes that are found in the chloroplasts of plants; these comprise the so-called ‘energy-conserving’ membranes. Part 1 of this book focuses on fundamental bioenergetic principles. In Part 2, we review our current understanding of the molecular (and increasingly atomic) structure and mechanisms of the protein complexes catalysing these processes, whereas in Part 3 we cover the explosive growth of mammalian cellular bioenergetics, in which the knowledge gained through the study of bioenergetics has become centre stage in investigations of the physiology and pathology of the eukaryotic cell.
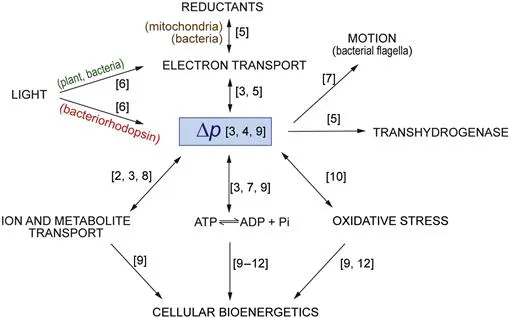
‘Bioenergetics’ originated in the quest to understand how oxidation reactions, in the form of the passage of electrons through coenzymes and proteins associated with these energy-conserving membranes, as well as photon capture in photosynthetic systems, could be coupled to the synthesis of ATP, the dominant common energy currency of the cell. The close similarity between the mechanisms and components involved in oxidative and photophosphorylation, as these processes are known respectively, allows them to be studied together. Attempts to relate the mechanism of oxidative phosphorylation to the ATP synthesising reactions in the glycolytic pathway were unsuccessful, and it turned out that this coupling was achieved via ion gradients across membranes in what became known as the chemiosmotic mechanism. It has further emerged over the years that these ion gradients, and hence the chemiosmotic mechanism, explain the coupling between a variety of other processes in cells, including how various species, from sugars and metabolites to proteins, are moved across these membranes (Figure I.1). The fundamentals of the chemiosmotic mechanism are frequently still confused today, 50 years after the emergence of the theory. One of our aims here, in the fourth edition of this book, is to continue to explain what this theory really implies as opposed to the oversimplified and frequently misleading accounts that can be found in many textbooks.
Figure I.1 Pathways of energy transduction.
The protonmotive force, Δp, interconnects multiple forms of energy. Numbers in square brackets refer to chapters in which pathways are discussed.
The basic principles are wonderfully simple; indeed, the ‘electrical circuit’ analogy we emphasise, with its ‘voltage’ and ‘current’ terms, continues to be useful at a research level. We continue to emphasise the importance of understanding the basic principles of thermodynamics applied to bioenergetic systems. We cover these in Chapter 3. It must be remembered that although thermodynamics can never prove a mechanism, it is ruthless in disproving energetically impossible ones. Particularly in the context of cellular bioenergetics, failure to grasp these key concepts is still disturbingly common. A word of caution, however: the treatment of thermodynamics in Chapter 3 is somewhat unconventional. Students preparing for examinations should check with their lecturers whether to adopt this simpler and more logical system, or to retain the classical approach based on ‘standard states’ and ‘standard free energies’.
![]()
1
Chemiosmotic Energy Transduction
1.1 The Chemiosmotic Theory: Fundamentals
Although some ATP synthesis is catalysed by soluble enzyme systems—for example, phosphoglycerate kinase in the glycolytic pathway—the large majority is generated by membrane-bound enzyme complexes that are restricted to a particular class of membrane. These ‘energy-transducing’ membranes include the plasma membrane of simple prokaryotic cells such as respiratory or photosynthetic bacteria, the inner membrane of mitochondria, and the thylakoid membrane of chloroplasts (Figure 1.1). These membranes have a related evolutionary origin because chloroplasts and mitochondria are commonly thought to have evolved from a symbiotic relationship between a primitive, nonrespiring eukaryotic cell and an invading prokaryote. The membranes not only catalyse ATP synthesis but also control the transport of ions and metabolites and the oxidation state in their environment. The mechanism of ATP synthesis and ion transport associated with these diverse membranes is sufficiently related, despite the differing natures of their primary energy sources, to form the core of classical bioenergetics.
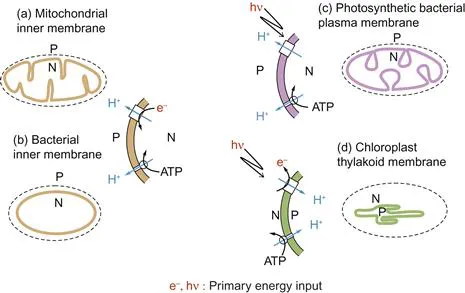
Figure 1.1 Energy-transducing membranes contain pairs of proton pumps with the same orientation.
In each case, the primary pump utilising either electrons (e−) from oxidations or driven by photons (hν) pumps protons from the N (negative) compartment to the P (positive) compartment. The photosynthetic bacterial photon-driven proton pump involves an electron transport cycle (not shown), whereas in addition to pumping protons, the chloroplast pumps electrons ‘uphill.’ Note that the ATP synthase in each case is shown acting in the direction of ATP hydrolysis, when it would also pump protons from the N- to the P-phase. The outer bounding membranes (dashed) do not participate in energy transduction.
Energy-transducing membranes possess a number of distinguishing features. Each membrane has embedded within it two distinct types of proton pump. The nature of the primary proton pump depends on the energy source used by the membrane. In the case of mitochondria or respiring bacteria, an electron transfer chain (Chapter 5) catalyses the energetically ‘downhill’ transfer of electrons from substrates to final acceptors such as O2 and uses this energy to generate an electrochemical gradient of protons. The term electrochemical is important and tells us that the energy gradient has electrical and chemical components (discussed later). Photosynthetic bacteria exploit the energy available from the absorption of quanta of visible light to generate a proton electrochemical gradient, whereas chloroplast thylakoids utilise two photon capture processes in series to generate the gradient and to drive electrons ‘uphill’ from water to acceptors such as NADP+ (Chapter 6). The detailed topologies of the membranes differ, and to facilitate comparison it is a useful convention to define the side of the membrane to which protons are pumped as the P, or positive, side and the side from which they have originated as the N, or negative, side (Figure 1.1).
In contrast to the variety of primary proton pumps, all energy-transducing membranes contain a highly conserved secondary proton pump termed the ATP synthase or the H+-translocating ATPase (Chapter 7). If this pump were operating in isolation in a membrane, it would hydrolyse ATP to ADP and Pi (shorthand for the phosphate anion) and pump protons in the same direction as the primary pump (Figure 1.1). However, the essence of the chemiosmotic theory is that the primary proton pump generates a sufficiently large electrochemical gradient of protons to force protons back through the secondary pump so that it reverses and synthesises ATP from ADP and Pi (Figure 1.2). Note that metabolism (i.e., electron flow or ADP phosphorylation) within both the primary pump and the secondary pump is tightly coupled to proton translocation. Thus, it is generally accepted that there is no internal ‘slip’ within these pumps that would allow one to occur without the other.
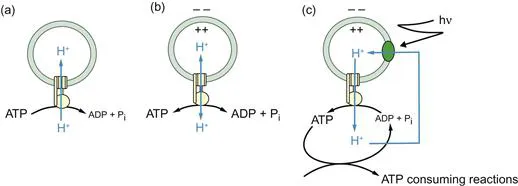
Figure 1.2 A hypothetical photosynthetic ‘thylakoid’ to demonstrate chemiosmotic coupling.
An ATP synthase complex is incorporated into a proton-impermeable phospholipid membrane such that the ATP binding site is on the outside. (a) ATP is added, the nucleotide starts to be hydrolysed to ADP + Pi, and protons are pumped into the vesicle lumen. As ATP is converted to ADP + Pi, the energy available from the hydrolysis steadily decreases, whereas the energy required to pump further protons against the electrochemical gradient that has already been established steadily increases. (b) Soon an equilibrium is attained. (c) If this equilibrium is now disturbed, for example, by removing ATP, the ATP synthase will reverse and attempt to re-establish the equilibrium by synthesising more ATP. Net synthesis, however, would be very small because the electrochemical gradient of protons would rapidly collapse and a new equilibrium would be established. For continuous ATP synthesis, a primary proton pump, driven in this example by photons (hν), is required to pump protons across the same membrane and replenish the gradient of protons. A proton circuit has now been established. This is what occurs across energy-conserving membranes: ATP is continuously removed for cytoplasmic ATP-consuming...