Ordered Porous Solids
Recent Advances and Prospects
Valentin Valtchev, Svetlana Mintova, Michael Tsapatsis, Valentin Valtchev, Svetlana Mintova, Michael Tsapatsis
- 800 páginas
- English
- ePUB (apto para móviles)
- Disponible en iOS y Android
Ordered Porous Solids
Recent Advances and Prospects
Valentin Valtchev, Svetlana Mintova, Michael Tsapatsis, Valentin Valtchev, Svetlana Mintova, Michael Tsapatsis
Información del libro
The developments in the area of ordered nanoporous solids have moved beyond the traditional catalytic and separation uses and given rise to a wide variety of new applications in different branches of chemistry, physics, material science, etc. The activity in this area is due to the outstanding properties of nanoporous materials that have attracted the attention of researchers from different communities. However, recent achievements in a specific field often remain out of the focus of collaborating communities. This work summarizes the latest developments and prospects in the area of ordered porous solids, including synthetic layered materials (clays), microporous zeolite-type materials, ordered mesoporous solids, metal-organic-framework compounds (MOFs), carbon, etc. All aspects, from synthesis via comprehensive characterization to the advanced applications of ordered porous materials, are presented. The chapters are written by leading experts in their respective fields with an emphasis on recent progress and the state of the art.
- Summarizes the latest developments in the field of ordered nanoporous solids
- Presents state-of-the-art coverage of applications related to porous solids
- Incorporates 28 contributions from experts across the disciplines
Preguntas frecuentes
Información
Chapter 1. A New Family of Mesoporous Oxides—Synthesis, Characterisation and Applications of TUD-1
Contents
- Introduction 4
- MCM-41 and FSM-16 5
- TUD-1 6
- Redox Metal Incorporation into Siliceous TUD-1 Framework 10
- Ti-TUD-1 10
- Fe-TUD-1 11
- Co- and Cr-TUD-1 14
- Ce-TUD-1 17
- Zr-TUD-1 18
- Al2 O3 -TUD-1 and Al-TUD-1 19
- TUD-1 as Potential Drug Carriers 21
- Particle Incorporation 22
- Zeolite Beta 22
- ITQ-2—Delaminated zeolite 26
- Conclusion 28
- References 29
Keywords
Mesoporous oxidesNanoparticlesComposite materialsSynthesisCatalysisAbbreviations
CTMACetyltrimethylammoniumFTIRFourier Transform InfraredMPVMeerwein-Ponndorf-VerleyNMRNuclear Magnetic ResonanceSMPOStyrene Monomer Propylene OxideTBHPtert-ButylhydroperoxideTEATriethanolamineTEAOHTetraethylammonium hydroxideTEOSTetraethoxysilaneTOFTurnover frequency(HR) TEM(High Resolution) Transmission Electron MicroscopyUV-visUltraviolet-Visible SpectroscopyXRDX-ray Diffraction
1. Introduction
2. MCM-41 and FSM-16
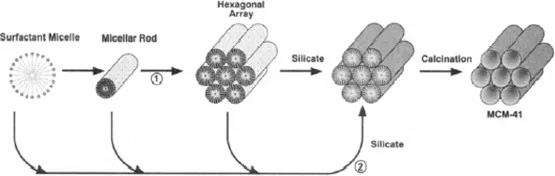
Figure 1.1. Possible mechanistic pathways for the formation of MCM-41: (1) liquid crystal phase initiated and (2) silicate anion initiated.