![]() Section 1: Basics
Section 1: Basics![]()
Chapter 1
What is Organometallic Chemistry
Hiroshi Nakazawaa
a Osaka City University Osaka, Japan
1.1 Organic Chemistry and Inorganic Chemistry
The development of chemistry has been impressive and many of the recent exciting advances have occurred at the interface of the traditional fields, resulting in the blurring of the areas of organic and inorganic chemistry. However, during the period when chemistry was developing, a distinction was made between compounds that were synthesized by living things and those that were not, leading to the branching of the science. The former and latter were termed respectively organic and inorganic chemistry. However, following Friedrich Wöhler's preparation of urea (an organic compound) artificially in 1828, it became possible to synthesize by human endeavor compounds which hitherto had been thought to be preparable only by biological systems. Along with that, the original meaning of “organic compounds” was lost, and the field has now become established in terms of carbon-centered chemistry, though the term is still used even today. Thus, the ideas that one 2s orbital and three 2p orbitals play important roles in organic chemistry and that the number of valence electrons around a carbon atom is eight, as embodied in the octet rule, are valid. In addition, sp3, sp2, and sp hybridizations reasonably explain the geometries around the carbon atom, such as tetrahedral, trigonal planar and linear. In contrast, inorganic chemistry became a general term referring to all chemical compounds except organic compounds. Thus, the elements included under “inorganic chemistry” comprise almost all the elements of the periodic table, and the orbitals used in discussing their bonding and properties include additionally d and f orbitals, thus leading to many compounds that do not obey the octet rule.
1.2 Coordination Chemistry
Among inorganic compounds, those containing transition metal atoms are especially complicated in terms of composition and structure. Compared to organic chemistry in which carbon may be considered to have 4 hands for making bonds, transition metals show variable numbers of bonding hands – from 1 to 10 – and thus seem complicated in comparison. The term “complex” in transition metal complex is derived from “complicated”.
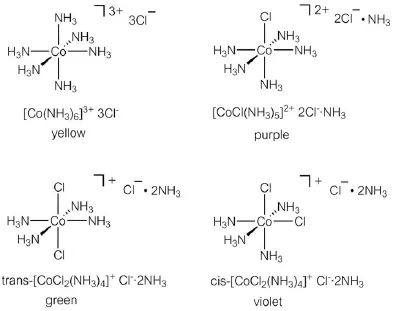
In the late 19th century, many complicated compounds involving a transition metal (transition metal complexes) were prepared and isolated. It was fortunate for transition metal chemistry that most of those complexes were colored. Even in the late 19th century, when spectroscopy was not yet popular, just by looking at the colors of the synthesized compounds, it could be judged whether they were the same or different. For example, the material represented by the composition CoCl3(NH3)6 appears yellow, violet, green and purple depending on the synthetic method. It was apparent that different compounds could still have the same composition. This could not be explained by the common sense of organic chemistry at that time.
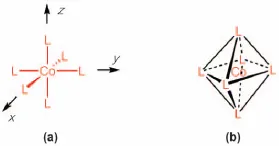
It was Alfred Werner who showed how to explain such a complicated compound reasonably. He started from the common sense of the time that “each element has a fixed valence” and introduced the idea that “the element can take different valences”. For example, cuprous chloride (CuCl) and cupric chloride (CuCl2) are both possible forms of copper chloride. Until then, it was thought that copper had 2 bonding hands, and thus CuCl2 was represented as Cl–Cu–Cl and CuCl as Cl–Cu–Cu–Cl. Werner, on the other hand, suggested that Cu in CuCl had 1 bonding hand and so the compound should be represented as Cu–Cl, and Cu in CuCl2 had 2 bonding hands and should be shown as Cl–Cu–Cl. Looking at the compound formulated as CoCl3(NH3)6 shown above, there are 9 atoms (Cl) or groups of atoms (NH3) other than Co. Co is considered to have 6 bonding hands, so the remaining 3 atoms or groups of atoms have no bonds with Co. An atom or group of atoms directly bonded to a metal is called a “ligand”. Werner's sharp insight revealed a spatially clear image of these 6 ligands and provided for a discussion of stereochemistry. Co is placed at the origin of the orthogonal coordinate system and six atoms (groups of atoms) are arranged in the directions of the x, -x, y, -y, z, and -z axes to form an octahedral molecular structure (Figure 1.1). Figure 1.2 shows the possible arrangements of CoCl3(NH3)6. Considering the stereochemistry of an octahedral structure, it is possible to explain the existence of geometric isomers, such as trans and cis, in this complex, and thus that compounds of different color can be formed despite having the same composition.
Figure 1.1 (a) Configuration of CoL6. (b) When connecting the six ligands (L) surrounding Co, it becomes an octahedron. There are no actual bonds between L and L.
Figure 1.2 Structures and colors of complexes formulated as CoCl3(NH3)6.
[PtCl2(NH3)2] has two isomers. If Pt takes a planar square structure, it can be understood that trans and cis isomers exist (Figure 1.3). cis-[PtCl2(NH3)2] is known as cisplatin and is effective as an anticancer drug.
Figure 1.3 Two isomers of square planar [PtCl2(NH3)2].
It was in 1893 that Werner proposed his revolutionary coordination theory. He was just 27 years old at the time. The chemical establishment of the time found his proposals hard to accept. However, the validity of Werner's coordination theory was proved by the accumulation of experimental results, and for this achievement, Werner was awarded the Nobel Prize in chemistry in 1913.
In coordination chemistry, the bond between metal and ligand is considered as follows. The ligand donates a lone pair of electrons to the empty d orbital of the transition metal to form a bond. The bond formed in this way is called a dative (or coordinate) bond. That is, the transition metal, as an electron pair acceptor, is a Lewis acid and the ligand, as an electron pair donor...