Emulsions
Tharwat F. Tadros
- 242 páginas
- English
- ePUB (apto para móviles)
- Disponible en iOS y Android
Emulsions
Tharwat F. Tadros
Información del libro
Chapter 1 General Introduction
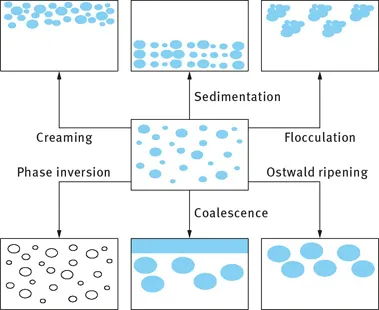
Definition of emulsions and the role of the emulsifier. Classification based on the nature of the emulsifier. Classification based on the structure of the system. General instability problems with emulsions: creaming/sedimentation, flocculation, Ostwald ripening, coalescence and phase inversion. Importance of emulsions in various industrial applications.
Chapter 2 Thermodynamics of Emulsion Formation and Breakdown
Application of the second law of thermodynamics for emulsion formation: Balance of energy and entropy and non-spontaneous formation of emulsions. Breakdown of the emulsion by flocculation and coalescence in the absence of an emulsifier. Role of the emulsifier in preventing flocculation and coalescence by creating an energy barrier resulting from the repulsive energies between the droplets.
Chapter 3 Interaction Forces between Emulsion Droplets
Van der Waals attraction and its dependence on droplet size, Hamaker constant and separation distance between the droplets. Electrostatic repulsion resulting from the presence of electrical double layers and its dependence on surface (or zeta) potential and electrolyte concentration and valency. Combination of the van der Waals attraction with double layer repulsion and the theory of colloid stability. Steric repulsion resulting from the presence of adsorbed non-ionic surfactants and polymers. Combination of van der Waals attraction with steric repulsion and the theory of steric stabilisation.
Chapter 4 Adsorption of Surfactants at the Oil/Water Interface
Thermodynamic analysis of surfactant adsorption and the Gibbs adsorption isotherm. Calculation of the amount of surfactant adsorption and area per surfactant molecule at the interface. Experimental techniques for measuring the interfacial tension.
Chapter 5 Mechanism of Emulsification and the Role of the Emulsifier
Description of the factors responsible for droplet deformation and its break-up. Role of surfactant in preventing coalescence during emulsification. Definition of the Gibbs dilational elasticity and the Marangoni effect in preventing coalescence.
Chapter 6 Methods of Emulsification
Pipe flow, static mixers and high speed stirrers (rotor-stator mixer). Laminar and turbulent flow. Membrane emulsification. High pressure homogenisers and ultrasonic methods.
Chapter 7 Selection of Emulsifiers
The hydrophilic-lipophilic-balance (HLB) and its application in surfactant selection. Calculation of HLB numbers and the effect of the nature of the oil phase. The phase inversion temperature (PIT) method for emulsifier selection. The cohesive energy ratio method for emulsifier selection.
Chapter 8 Creaming/Sedimentation of Emulsions and its prevention
Driving force for creaming/sedimentation: effect of gravity, droplet size and density difference between the oil and continuous phase. Calculation of the rate of creaming/sedimentation in dilute emulsions. Influence of increase of the volume fraction of the disperse phase on the rate of creaming/sedimentation. Reduction of creaming/sedimentation: Balance of the density of the two phases, reduction of droplet size and effect of addition of ''thickeners'.
Chapter 9 Flocculation of Emulsions and its Prevention
Factors affecting flocculation. Calculation of fast and slow flocculation rate. Definition of stability ratio and its dependence on electrolyte concentration and valency. Definition of the critical coagulation concentration and its dependence on electrolyte valency. Reduction of flocculation by enhancing the repulsive forces.
Chapter 10 Ostwald Ripening and its Reduction
Factors responsible for Ostwald ripening: difference in solubility between small and large droplets and the Kelvin equation. Calculation of the rate of Ostwald ripening. Reduction of Ostwald ripening by incorporation of a small amount of highly insoluble oil. Reduction of Ostwald ripening by the use of strongly adsorbed polymeric surfactant and enhancement of the Gibbs elasticity.
Chapter 11 Emulsion Coalescence and its Prevention
Driving force for emulsion coalescence: Thinning and disruption of the liquid film between the droplets. The concept of disjoining pressure for prevention of coalescence. Methods for reduction or elimination of coalescence: Use of mixed surfactant films, use of lamellar liquid crystalline phases and use of polymeric surfactants.
Chapter 12 Phase Inversion and its Prevention
Distinction between catastrophic and transient phase inversion. Influence of the disperse volume fraction and surfactant HLB number. Explanation of the factors responsible for phase inversion.
Chapter 13 Characterisation of Emulsions
Measurement of droplet size distribution: Optical microscopy and image analysis. Phase contrast and polarising microscopyDiffraction methods. Confocal laser microscopy. Back scattering methods
Chapter 14 Industrial Application of Emulsions
14.1 Application in Pharmacy
14.2 Application in Cosmetics
14.3 Application in Agrochemicals
14.4 Application in Paints
14.5 Application in the Oil Industry
Preguntas frecuentes
Información
1Emulsions: Formation, stability, industrial applications
1.1General introduction
| Nature of emulsifier | Structure of the system |
| –Simple molecules and ions | –Nature of internal and external phase: O/W, W/O |
| –Nonionic Surfactants | –Nanoemulsions |
| –Ionic surfactants | –Micellar emulsions (microemulsions) |
| –Surfactant mixtures | –Macroemulsions |
| –Nonionic Polymers | –Bilayer droplets |
| –Polyelectrolytes | –Double and Multiple Emulsions |
| –Mixed polymers and surfactants | –Mixed emulsions |
| –Liquid crystalline phases | |
| –Solid particles |
1.2Nature of the Emulsifier
1.3Structure of the system
1.4Breakdown processes in emulsions