![]()
Ronco C (ed): Expanded Hemodialysis – Innovative Clinical Approach in Dialysis.
Contrib Nephrol. Basel, Karger, 2017, vol 191, pp 84–99 (DOI: 10.1159/000479258)
_______________
Solute Transport in Hemodialysis: Advances and Limitations of Current Membrane Technology
William R. Clarka · Dayong Gaob · Mauro Neric · Claudio Roncoc, d
aDavidson School of Chemical Engineering, Purdue University, West Lafayette, IN, and bDepartment of Mechanical Engineering, University of Washington, Seattle, WA, USA; cInternational Renal Research Institute of Vicenza (IRRIV) and dDepartment of Nephrology, San Bortolo Hospital, Vicenza, Italy
______________________
Abstract
Extracorporeal therapy for end-stage renal disease is now provided to more than three million patients globally. Nearly all treatments are performed with filters containing hollow fiber membranes, removing solutes and water by diffusion, convection, and ultrafiltration. In this review, we will provide a detailed quantitative analysis of the transport processes involved in different hemodialysis (HD) therapies. We will also report some technical aspects of hollow fiber membranes and filters composed of them along with the mechanisms of solute and water removal for such devices. Diffusive mass transfer will be assessed according to the three major aspects of a hollow fiber filter (blood compartment, membrane, and dialysate compartment). With regard to convective transport, the importance of internal filtration as a solute removal mechanism in high-flux HD will be highlighted, along with the critical role that blood/membrane interactions assume in filtration-based therapies.
© 2017 S. Karger AG, Basel
Introduction
Extracorporeal therapy for end-stage renal disease is now provided to more than three million patients globally. Nearly all treatments are performed with filters containing hollow fiber membranes, removing solutes and water by diffusion, convection, and ultrafiltration. The routine use of these therapies belies their technical complexity and dynamic nature. In this review, we will provide a detailed quantitative analysis of the transport processes involved in different hemodialysis (HD) therapies. We will also report some technical aspects of hollow fiber membranes and filters composed of them along with the mechanisms of solute and water removal for such devices.
Hollow Fiber Membranes
Solute Characteristics
Uremic retention molecules represent a spectrum of substances, normally cleared by the native kidney, whose concentration in blood increases significantly as renal function decreases. Several of these molecules are efficiently removed by the current HD techniques, although limitations to solute transport may occur due to the intrinsic characteristics of the molecule, including molecular weight, molecular radius, hydrophicity, electrical charges, and protein binding. As discussed below, diffusive transport rates tend to decrease with increasing molecular weight or radius. This limitation has two basic determinants: the diffusion coefficient of the molecule and the possible restriction imposed by the membrane. The combination of diffusion and convection in HD is intended to overcome this limitation and optimize solute removal in the widest possible molecular weight spectrum.
Hollow Fiber Design
An individual hollow fiber can be viewed as a solid cylinder in which the central region has been removed (“cored out”) to form an annular blood compartment [1]. The process of manufacturing (“spinning”) a hollow fiber membrane is complex, and incorporates aspects of a broad array of scientific disciplines, including polymer chemistry, thermodynamics, reaction kinetics, and chemical engineering [2]. Although clear differences exist among the different types of membranes used clinically, a number of common features apply. From a structural perspective, most hollow fibers have a relatively standard inner (blood compartment) diameter (approximately 180–220 µm) and length (20–24 cm). These parameters are dictated essentially by the operating conditions existing during HD and are the result of a compromise between opposing forces. On one hand, a relatively small hollow fiber inner diameter is desirable because it provides a short diffusive distance for solute mass transfer. At a given blood flow rate, a smaller bore fiber also creates a higher shear rate, resulting in greater attenuation of blood-side boundary layer effects [3]. However, the degree to which the inner diameter can be reduced is limited. Flow along the length of a cylinder (i.e., axial flow) of fluids such as water and blood (i.e., Newtonian fluids) is governed by the Hagen-Poiseuille law [4]:
where QB is the blood flow rate, ΔP is the axial pressure drop, µ is the blood viscosity, L is the fiber length, and r is the hollow fiber radius. A specific application of this equation is blood flow from the arterial to venous end of a hollow fiber membrane during HD. A more general form of equation 1 is:
From equations 1 and 2, the resistance (R) to blood flow is:
Due to the inverse relationship between flow resistance and r4, a small decrease in the inner diameter of the hollow fiber induces a large increase in flow resistance. Equation 3 also demonstrates that increases in fiber length and hematocrit (Hct; through an effect on blood viscosity) are associated with an increase in flow resistance. In turn, as indicated by equation 2, an increase in flow resistance results in a proportional increase in axial pressure drop at constant blood flow rate.
Surface Area
For a particular hollow fiber, the inner annular surface represents the nominal blood compartment surface and is theoretically the maximum area available for blood contact. For the entire group of fibers comprising a filter (i.e., the fiber bundle), the total nominal surface depends on the fiber length, inner diameter, and overall number of fibers, the latter of which varies generally from approximately 9,000 to 16,000. For calculation of dialyzer membrane surface area, the surface area of an individual fiber can be estimated by using the formula for a cylinder:
Based on assumed values of r = 100 µm (10-4 m) and L = 24 cm (0.24 m), the surface area of an individual fiber can be calculated as 1.51 × 10-4 m2. For a relatively large filter (n = 14,000):
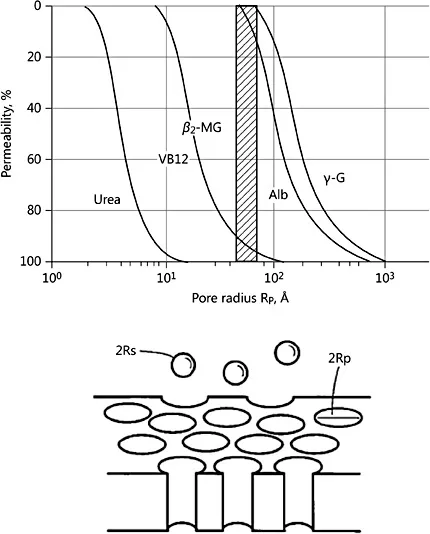
Fig. 1. Idealized membrane model in which all pores have the same radius and are cylinders perpendicular to the blood and dialysate flows. Reprinted with permission from Takeyama and Sakai [5].
Effect of Membrane Pore Characteristics on Water Permeability
To provide a rough quantitative estimate of parameters related to pore characteristics of a hollow fiber membrane, a straight cylindrical pore model can be used as a first approximation. As shown in Figure 1 [5], this model assumes that the membrane’s pores have the same radius (rp), which is larger than the radius (rs) of the hypothetical solute given. In addition, the directional orientation of the parallel cylinders is assumed to be perpendicular to the flow of blood and dialysate. As noted above, the Hagen-Poiseuille law governs the fluid flow through cylindrical tubes in many situations, applying also to transmembrane ultrafiltrate flow through the pores in this model. As such, the rate of ultrafiltrate flow is directly related to the fourth-power of the pore radius (i.e., r4) at constant transmembrane pressure.
A more sophisticated approach accounts for the fact that membrane pores are not “perfect” cylinders [6]:
In this equation, Kf is the hydraulic permeability of the membrane, n is the number of pores per unit area (i.e., pore density), r is the pore radius, τ is a factor accounting for pore tortuosity, µ is the ultrafiltrate viscosity, and Δx is ...