RICHARD B. M. SCHASFOORT
Medical Cell BioPhysics Group (MCBP), MIRA Institute, University of Twente, P.O. Box 217, 7500 AE Enschede, The Netherlands
Email:
[email protected]1.1 Introduction to Surface Plasmon Resonance
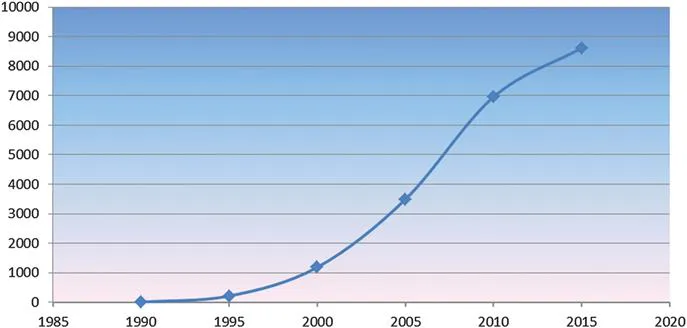
During the years following the introduction of the first commercial surface plasmon resonance (SPR) instrument (Biacore) in 1990, the number of publications that include data collected from commercial biosensors increased to more than 20 000 papers by 2016 (PubMed data), as shown in Figure 1.1.
Figure 1.1 SPR papers in PubMed: total >20 000. 1990–1995: 217 articles; 1995–2000: 1190 articles; 2000–2005: 3493 articles; 2005–2010: 6958 articles; 2010–2015: 8617 articles.
Not only the number of publications but also advances in the technology led to an improvement in detection sensitivity by roughly 100-fold up to 10−8 RIU (refractive index unit) or 0.01 RU (resonance or response unit). The range of affinity and kinetic data that can be determined has been extended at least 200-fold as a consequence of the increased sensitivity and due to improvements in data analysis. The number of independent channels or spots grew from four channels in 1990 (Biacore) to at least 192 flow-controlled spots in the new IBIS MX96 imaging instrument and more than 10 000 drop-spotted ligands in SPR imaging instruments from various manufacturers (e.g. Plexera). The carboxymethylated dextran surface introduced in 1990,1 still the first choice for many applications, has been complemented with a range of other surfaces (see Chapter 6). Systems for dedicated applications have been introduced by various manufacturers as complements to all-purpose research instrumentation,2 and the impact of SPR biosensors on biomolecular interaction studies is growing continuously. With improved experimental design and advanced data analysis methods, high-quality data for the determination of kinetic parameters of biomolecular interaction phenomena can be obtained. These data promise additional insights not only into the affinity of biomolecular pairs but also into the mechanisms of molecular binding events, which will be important for function–regulatory protein interaction studies in order to unravel the exciting processes in living species.
1.2 What is a Biosensor?
The term biosensor was introduced around 1975, relating to the exploitation of transducer principles for the direct detection of biomolecules at surfaces. Currently the most prominent example of a biosensor is the glucose sensor, reporting glucose concentration as an electronic signal, e.g. based on a selective enzymatic process. According to the current definition, in biosensors the recognition element (ligand) of the sensor or the analyte should originate from a biological source.
Biosensors are analytical devices comprising a biological element (tissue, microorganism, organelle, cell receptor, enzyme, antibody) and a physicochemical transducer. Specific interaction between the target analyte and the biological material produces a physicochemical change detected by the transducer. The transducer then yields an analog electronic signal proportional to the amount (concentration) of a specific analyte or group of analytes.
Anthony P. F. Turner (Editor, Biosensors and Bioelectronics)
Application of SPR-based sensors to biomolecular interaction monitoring was first demonstrated in 1983 by Liedberg et al.3 A historical overview of the use of the phenomenon for biosensor applications is given in Chapter 2. To understand the excitation of surface plasmons, let us start with a simple experiment.
1.2.1 A Simple Experiment
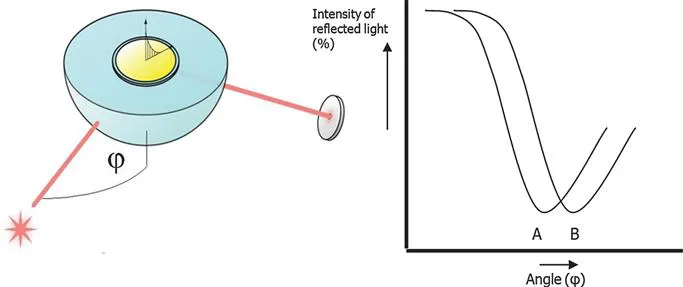
Consider the experimental set-up depicted in Figure 1.2. When polarized light is shone through a prism on a sensor chip with a thin metal film on top, the light will be reflected by the metal film acting as a mirror. On changing the angle of incidence and monitoring the intensity of the reflected light, one observes that the intensity of the reflected light passes through a minimum (Figure 1.2, line A). At this angle of incidence, the light will excite surface plasmons, inducing surface plasmon resonance, causing a dip in the intensity of the reflected light. Photons of p-polarized light can interact with the free electrons of the metal layer; inducing a wave-like oscillation of the free electrons, thereby reducing the reflected light intensity.
Figure 1.2 Schematic experimental set-up of surface plasmon resonance excitation. A sensor chip with a gold coating is placed on a hemisphere (or prism). Polarized light shines from the light source (star) on the sensor chip. Reflected light intensity is measured in the detector (disk). At a certain angle of incidence (φ), excitation of surface plasmons occurs, resulting in a dip in the intensity of the reflected light (A). A change of refractive index at the surface of the gold film, will cause an angle shift from A to B.
The angle at which the maximum loss of the reflected light intensity occurs is called resonance angle or SPR-dip. The SPR-dip angle is dependent on the optical characteristics of the system, e.g. on the refractive indices of the media on both sides of the metal, usually gold, and are explained in detail in Chapter 2. Whereas the refractive index at the prism side does not change, the refractive index in the immediate vicinity of the metal surface will change when accumulated mass (e.g. proteins) adsorb on the thin gold layer. Hence the SPR conditions are changing and the real-time shift of the SPR angle is suited to provide information on the kinetics of, e.g., protein adsorption on the surface.
1.2.2 From Dip to Real-time Measurement
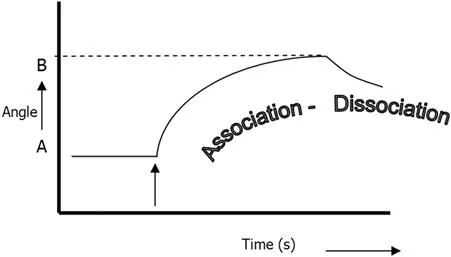
Surface plasmon resonance is an excellent method for monitoring changes in the refractive index in the near vicinity of the metal surface. When the refractive index changes, the angle at which the intensity minimum is observed will shift as indicated in Figure 1.2, where line A depicts the original plot of reflected light intensity versus incident angle, and B is the plot after the change in refractive index. SPR is not only suited to measure the difference between these two states, it can also monitor the change in time, if one follows in time the shift of the resonance angle at which the dip is observed. Figure 1.3 depicts the shift of the dip in time, a so-called sensorgram. If this change is due to a biomolecular interaction, the kinetics of the interaction can be studied in real time.
Figure 1.3 A sensorgram: the angle at which the dip is observed versus time. First, no change occurs at the sensor and a baseline is measured with the dip at SPR angle (A). After injection of the sample (arrow), biomolecules will adsorb on the surface, resulting in a change in refractive index and a shift of the SPR angle to position B (association). The adsorption–desorption process can be followed in real time and the amount of adsorbed species can be determined. Dissociation of the analyte bound to the ligand occurs when the sample containing the analyte is exchanged again with the system buffer.
SPR sensors investigate only in a very limited vicinity in a fixed volume at the metal surface. The penetration depth of the electromagnetic field (so-called evanescent field) at which a signal is observed typically does not exceed a few hundred nanometers, decaying exponentially with the distance from the metal layer at the sensor surface. The penetration depth of the evanescent field is a function of the wavelength of the incident light, as explained in Chapter 2.
SPR sensors lack intrinsic selectivity: all refractive index changes in the evanescent field will result in a change of the signal. These changes can be due to a refractive index difference of the medium, e.g. a change in the buffer composition or concentration, or temperature effects; non-specific and specific adsorption of material on the sensor surface can also cause refractive index changes. The amount of adsorbed species can be determined after injection of the original baseline buffer as shown in Figure 1.3. To allow selective detection at an SPR sensor, its surface needs to be modified with ligands suited for selective capturing of the target compounds (the analyte) but whic...