Gels Handbook: Fundamentals, Properties, Applications (In 3 Volumes)
Fundamentals, Properties and Applications(In 3 Volumes)Volume 1: Fundamentals of HydrogelsVolume 2: Applications of Hydrogels in Regenerative MedicineVolume 3: Application of Hydrogels in Drug Delivery and Biosensing
Utkan Demirci, Ali Khademhosseini
- 1,172 páginas
- English
- ePUB (apto para móviles)
- Disponible en iOS y Android
Gels Handbook: Fundamentals, Properties, Applications (In 3 Volumes)
Fundamentals, Properties and Applications(In 3 Volumes)Volume 1: Fundamentals of HydrogelsVolume 2: Applications of Hydrogels in Regenerative MedicineVolume 3: Application of Hydrogels in Drug Delivery and Biosensing
Utkan Demirci, Ali Khademhosseini
Información del libro
Hydrogels are made from a three-dimensional network of cross linked hydrophilic polymers or colloidal particles that contain a large fraction of water. In recent years, hydrogels have attracted significant attention for a variety of applications in biology and medicine. This has resulted in significant advances in the design and engineering of hydrogels to meet the needs of these applications. This handbook explores significant development of hydrogels from characterization and applications. Volume 1 covers state-of-art knowledge and techniques of fundamental aspects of hydrogel physics and chemistry with an eye on bioengineering applications. Volume 2 explores the use of hydrogels in the interdisciplinary field of tissue engineering. Lastly volume 3 focuses on two important aspects of hydrogels, that is, drug delivery and biosensing. Contains 50 colour pages.
Contents:
- Volume 1: Fundamentals of Hydrogels (Qi Wen [Worcester Polytechnic Institute, USA] and Yi Dong [OPKO Diagnostics, LLC, OPKO Health, Inc., USA])
- Natural Hydrogels (Shangyou-Tee [NTU, Singapore])
- Types and Chemistry of Synthetic Hydrogels (John Garner [Akina, Inc., USA] and Kinam Park [Purdue University, USA])
- Computational Nanomechanics of Hydrogels (Hossein Salahshoor and Nima Rahbar [Worcester Polytechnic Institute, USA])
- Mechanical Properties of Hydrogels (Anne S van Oosten, Peter A Galie and Paul A Janmey [University of Pennsylvania, USA])
- Hydrogel Architecture (Fariba Dehghani and Ali Fathi [The University of Sydney, Australia])
- Controlling Hydrogel Biodegradability (Wesley N Sivak, Danielle M Minteer, Bernd Lannau and Kacey G Marra [University of Pittsburgh, Pittsburgh, USA])
- Tailoring Hydrogel Adhesiveness to Cells, Proteins, and Bacteria (Silviya Petrova Zustiak [Saint Louis University, USA])
- Photo-Cross-Linking Methods to Design Hydrogels (Brian Amsden [Queen's University, Canada])
- Self-Assembling Hydrogels (Annada Rajbhandary and Bradley L Nilsson [University of Rochester, USA])
- Environment Responsive Hydrogels (Yanzhen Yin, Shufei Jiao [Qinzhou University, China], Chao Lang and Junqiu Liu [Jilin University, China])
- Volume 2: Applications of Hydrogels in Regenerative Medicine (Mohammad Reza Abidian [University of Houston, USA], Umut Atakan Gurkan [Case Western Reserve University, USA] and Faramarz Edalat [Emory University, USA])
- Hydrogels in Regenerative Medicine (Nesrin Hasirc, Cemile Kilic, Aylin Kömez, Gökhan Bahcecioglu and Vasif Hasirci [Middle East Technical University, Turkey])
- Determining Stem Cell Fate with Hydrogels (Aylin Acun, Andreana Panzo and Pinar Zorlutuna [University of Notre Dame, USA])
- Applications of Hydrogels in 3D Functional Tissue Models (Tamer Çırak, Tayfun Vural [Hacettepe University, Turkey], Doǧa Kavaz [International Cyprus University, Northern Cyprus] and Emir Baki Denkbaş [Hacettepe University, Turkey])
- Engineering Regenerative Dextran Hydrogels for Acute Skin Wound Healing (Jie Cheng, Ying Jin, Jeremy J Mao, Guoming Sun [Columbia University Medical Center, USA]) and David M Owens [Columbia University, USA])
- Application of Hydrogels in Ocular Tissue Engineering (Vipuil Kishore [Florida Institute of Technology, USA], Yunus Alapan [Case Western Reserve University, USA], Ranjani Iyer, Ryan Mclay [Florida Institute of Technology, USA] and Umut A Gurkan [Case Western Reserve University, USA and Louis Stokes Cleveland Veterans Affairs Medical Center, USA])
- Hydrogels in Bone Tissue Engineering: A Multi-Parametric Approach (Silvia M Mihaila, Rui L Reis, Alexandra P Marques and Manuela E Gomes [University of Minho, Portugal])
- Hydrogels in Intervertebral Disk (IVD) Repair (Cem Bayram [Aksaray University, Turkey], Murat Demirbilek and Emir Baki Denkbaş [Hacettepe University, Turkey])
- Hydrogels in Cartilage Tissue Engineering (Antonella Motta, Mariangela Fedel and Claudio Migliaresi [University of Trento, Italy])
- Application of Hydrogels for Tendon and Ligament Repair and Tissue Engineering (Matteo Stoppato, Friedrich von Flotow [Tufts University] and Catherine K Kuo [Tufts University and Tufts University School of Medicine])
- Hydrogels in Bone Tissue Engineering (Minh Khanh Nguyen, Julia E Samorezov and Eben Alsberg [Case Western Reserve University, USA])
- Hydrogels in Cardiac Tissue Engienering (Shauna M Dorsey and Jason A Burdick [University of Pennsylvania, USA])
- Application of Hydrogels in Heart Valve Tissue Engineering (Jesper Hjortnaes [University Medical Center Utrecht, Netherlands] and Frederick J Schoen [Brigham & Women's Hospital, Harvard Medical School, USA])
- Hydrogels in Vascular Tissue Engineering (Y J Blinder [Israel Institute of Technology, Israel and Harvard University, USA], D J Mooney [Harvard University, USA] and S Levenberg [Israel Institute of Technology, Israel])
- Use of Hydrogels in the Engineering of Lung Tissue (Joaquin Cortiella, Jean A Niles, Stephanie P Vega, Lissenya Argueta, Adriene Eastaway and Joan E Nichols [Weill Cornell Medical College, USA])
- Hydrogels for Hepatic Tissue Engineering (Kerim B Kaylan and Gregory H Underhill [University of Illinois at Urbana-Champaign, USA])
- Agarose Hydrogel Beads for Treating Diabetes (Nguyen Minh Luan, Yuji Teramura and Hiroo Iwata [Kyoto University, Japan])
- Hydrogels in Urogenital Applications (James Turner and Jiro Nagatomi [Clemson University, USA])
- Volume 3: Application of Hydrogels in Drug Delivery and Biosensing (Lifeng Kang [NUS, Singapore] and Sheereen Majd [University of Houston, USA]):
- Modeling Drug Release from Synthetic Hydrogels (Chien-Chi Lin [Indiana University-Purdue University Indianapolis, USA] and Han Shih [Purdue University, USA])
- Natural Polysaccharide-Based Hydrogels for Controlled Localized Drug Delivery (Roman P Blount, IV and Narayan Bhattarai [North Carolina A&T State University, USA])
- In Situ Crosslinked Hydrogels for Drug Delivery (Jin Woo Bae and Ki Dong Park [Ajou University, South Korea])
- Supramolecular Hydrogels for Drug Delivery (Prashant Sawant [RMIT, Australia])
- BioHybrid Hydrogels as Environment-Sensitive Materials for Systematic Delivery of Therapeutics (Sara Faraji Dana, Jing Pan [NUS, Singapore] and Maziar S Ardejani [King's College London, UK])
- Application of PEG in Drug Delivery System (Bramasta Nugraha [ETH Zürich, Switzerland and Roche Innovation Center Basel, Switzerland])
- Hydrogels as Actuators for Biological Applications (Hongrui Jiang and Difeng Zhu [University of Wisconsin Madison, USA])
- Advances in Smart Hydrogels for Biosensing Applications (Wei Wang, Jimmy Amin, and Zhiqiang Cao [Wayne State University, USA])
- 3D Cancer Models on Hydrogels (Imran Rizvi, Heather Gudejko, Emma Briars, Shazia Khan, Chun-Te Chiang, [Massachusetts General Hospital and Harvard Medical School, USA], Hamid El-Hamidi, Jonathan P Celli [University of Massachusetts Boston, USA] and Tayyaba Hasan [Massachusetts General Hospital and Harvard Medical School, USA])
- Hydrogel-Mediated Patterning of Cellular and Biomolecular Microarrays for Screening Assays and Biosensing (You Jung Kang, Soo Hyun Park, and Sheereen Majd [Pennsylvania State University, USA])
- Protein-Immobilized Hydrogel Microstructures for Optical Biosensing (Won-Gun Koh [Yonsei University, South Korea])
- Cell-Encapsulating Hydrogels for Biosensing (Pu Chen, Shuqi Wang, Fatih Inci, Sinan Güven, Savas Tasoglu and Utkan Demirci [Stanford University, USA])
Readership: Pharmaceutical researchers and bioengineers, material scientists, biologists, physicians and students with an interest in the field of tissue engineering and regenerative medicine.
Preguntas frecuentes
Información
Chapter 1
Natural Hydrogels
1.Introduction
1.1.Types and chemistry of natural hydrogels
1.2.Classify hydrogels based on their formation
2.Hydrogel Synthesis
2.1.Physical crosslink
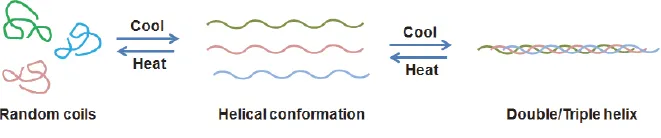
2.1.1.Heating or cooling process
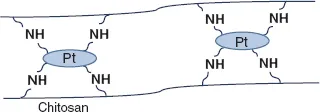
2.1.2.Ionic interaction
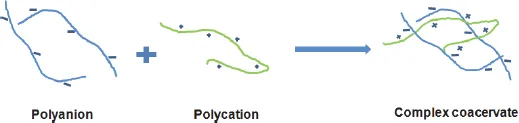
2.1.3.Complex coacervation