![]()
CHAPTER 1
Preformulation Studies
TREVOR M. JONES
1.1 Introduction
Discovering and developing new medicines is a long, complex and expensive process and the failure rate is high during the process. To minimise attrition it is essential, therefore, to understand the physicochemical characteristics of compounds or biological entities that are candidates for development into final products.
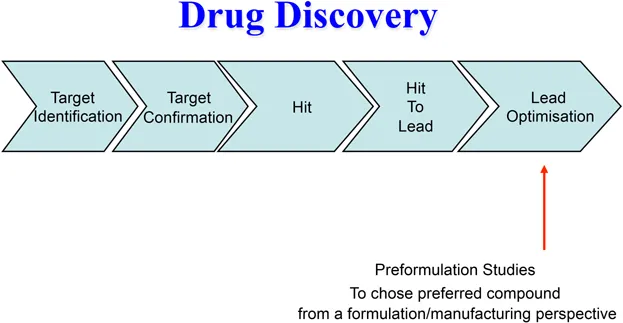
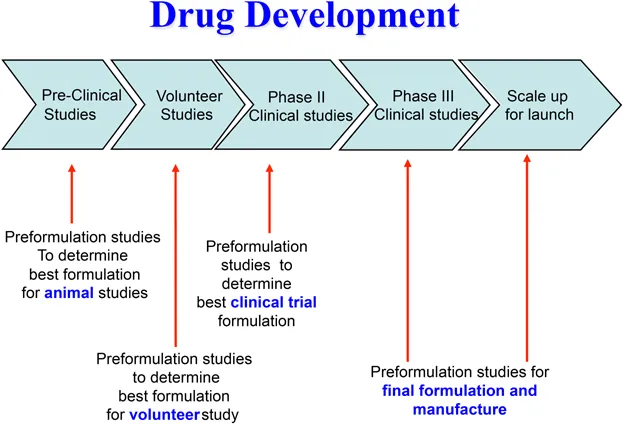
At various stages during the development of a new medical product the candidate drug must be formulated into a dosage form that is appropriate for the intended study e.g. in vitro screening using chemical, physicochemical or biological assays, pre-clinical in vitro laboratory safety tests, in vivo efficacy and safety studies in relevant animal species, first-in-human studies to determine the optimum drug to progress into clinical development, initial volunteer/patient studies and full-scale clinical trials (Figures 1.1 and 1.2).
Figure 1.1 Early stage preformulation studies.
Figure 1.2 Preformulation studies at various stages of development.
The nature and composition of the formulations will be different for each stage of development but the formulation chosen for full-scale clinical trials must, as far as possible, be the same as the product that is intended for marketing. Otherwise extensive clinical comparative trials may be required to demonstrate the similarity between the formulation used in the clinical trials and that proposed for subsequent marketing.
To ensure that the various formulations are optimised for their intended use, pre-formulation studies should be conducted not only to evaluate the characteristics of candidate drugs but also potential formulation excipients, and their interactions with drug substances, in order to select appropriate formulation ingredients. In addition, preformulation studies should assess the effect of possible conditions of preparation, manufacture and storage on stability, so as to give confidence that a reliable assessment of the candidate drug has been performed during development and in regular, post-marketing, use.
Data acquired from preformulation studies also forms an important basis for understanding the potential pharmacokinetics of a drug in humans and animals.
In addition, as the chosen product is scaled up in manufacture and/or further process development is carried out e.g. to use alternative equipment or technologies; preformulation data can be a useful source of information to understand the opportunities for and limitations to process change.
Furthermore, a number of the characteristics measured in preformulation studies can be used to predict the stability of the formulation during manufacture, transport and storage so as to determine the shelf life of the marketed product.
Preformulation studies can therefore be defined as; Laboratory studies to determine the characteristics of active substance and excipients that may influence formulation and process design and performance.
It has been described as “Learning before doing”.
1.2 Solubility
The aqueous and lipid solubility characteristics of a drug substance are of fundamental importance in determining whether it is capable of reaching sites of absorption, its interaction with putative therapeutic targets and its ultimate metabolism and excretion.
An assessment of solubility characteristics is, therefore, usually a starting point for preformulation studies.
1.2.1 Absolute (Intrinsic) Solubility
Using standard aqueous buffers the drug or excipient is vigorously stirred at a constant temperature, e.g. 37 °C, to achieve equilibrium, maximum (saturated) absolute solubility. For compounds with ionisable groups this equilibrium solubility of the unionised form is known as the intrinsic solubility.
Preformulation studies will start by measuring intrinsic solubility in a neutral, an acid and an alkaline environment; typically 0.1 M HCl, water and 0.1 M NaOH at 4 °C, 25 °C, 37 °C and an elevated temperature e.g. 50 °C.
These data can be recorded as the absolute (intrinsic) aqueous solubility at each pH and compared with data on known and related compounds.
The values obtained can provide insight into the state of the drug substance as it is subjected to a variety of different pH environment e.g. as it passes through the gastro-intestinal tract, circulates through various cellular, organ components, arterial and venous circulation and excretory fluids such as bile and urine.
In addition the solubility profile at different pH's can inform the type of the aqueous solvents that might potentially be used in formulations (e.g. parenteral injections, nasal or ophthalmic drops, oral solutions).
Furthermore, the information is useful to assess the possible effect that aqueous media used in dosage form manufacture, e.g. tablet wet granulation and film coating, may have on the compound.
1.2.2 Molecular Dissociation pKa
The aqueous solubility of a compound is dependent, inter alia, on its state of ionization, including the ratio of ionised to unionised moiety.
The degree of ionisation can be estimated using the Henderson–Hasselbach equation which for weak acidic compounds (HA) is
pKa = pH + log[HA]/[A−]
or in its rearranged form
pH = pKa + log[A−]/[HA]
where Ka is the ionisation constant of the dissociation constant.
And for weakly basic compounds (BH)
pKa = pH + log[BH+]/[B]
Or
pH = pKa + log[B]/[BH+]
pKa is obtained by measuring the pH changes of the substance in solution during potentiometric titration using either a weak base or a weak acid. When pH = pKa the compound is 50% ionised.
The pKa can be calculated from intrinsic solubility data; also measured using a variety of techniques e.g. conductivity, potentiometry and spectroscopy.
The pKa value provides a useful indication as to the region of the gastrointestinal tract in which the drug will be in either the ionised or unionised state and, hence, some indication of its possible absorption characteristics.
Importantly, however, the chemical nature and concentration of the counter ion conferring solubility e.g. chloride or hydrochloride can have a significant influence on solubility and this should be examined during preformulation studies; so as to choose an optimum compound e.g. base or cation, for further development.
1.2.3 Solubility in Various Solvents
In addition to determining the solubility characteristics in an aqueous environment it is also useful to obtain preliminary data on the solubility of the drug/excipient in non-aqueous solvents that might be used in formulations, e.g. topical ointments/liniments or oily injections, and to provide data that can be used to select solvents for manufacture of the active ingredient, e.g. extraction or crystallisation, and for the final formulation, e.g. tablet granulation.
Since there are many organic solvents that might be employed, preliminary preformulation studies should focus on a selection of solvents such as:
For Formulation
- Ethyl alcohol
- Glycerin
- Propylene glycol
- Arachis oil
- Ethyl oleate
- Liquid paraffin
For Manufacture
- Industrial methylated spirits
- Isopropyl alcohol
- Benzyl alcohol
- Polyethylene glycol
1.2.4 Solubility Rate (Dissolution)
Whilst a knowledge of intrinsic solubility is essential, the rate at which a drug or excipient dissolves in any particular medium is also important.
Solubility rate will depend on many factors, such as particle size; particle size distribution and particle porosity—and, hence, the surface area available, which is changing as dissolution occurs—the wettability of the particle surfaces, the nature of the dissolution fluid, its polarity, rheological properties and the degree of stirring or agitation during dissolution.
Therefore, initial preformulation studies s...