
eBook - ePub
Endodontic Microbiology
Ashraf F. Fouad, Ashraf F. Fouad
This is a test
Compartir libro
- English
- ePUB (apto para móviles)
- Disponible en iOS y Android
eBook - ePub
Endodontic Microbiology
Ashraf F. Fouad, Ashraf F. Fouad
Detalles del libro
Vista previa del libro
Índice
Citas
Información del libro
Endodontic Microbiology, Second Edition presents a comprehensive reference to the microbiology, pathogenesis, management, and healing of endodontic pathosis, emphasizing the importance of biological sciences in understanding and managing endodontic disease and its interaction with systemic health.
- Provides a major revision to the first book to focus on the problems related to microbes in the root canal and periapical tissues
- Updates current knowledge in endodontic pathosis, especially regarding next generation sequencing and microbial virulence
- Presents useful diagrams, images, radiographs, and annotated histological images to illustrate the concepts
- Emphasizes the importance of biological science in understanding and managing endodontic disease
- Includes contributions from the leading researchers and educators in the field
Preguntas frecuentes
¿Cómo cancelo mi suscripción?
¿Cómo descargo los libros?
Por el momento, todos nuestros libros ePub adaptables a dispositivos móviles se pueden descargar a través de la aplicación. La mayor parte de nuestros PDF también se puede descargar y ya estamos trabajando para que el resto también sea descargable. Obtén más información aquí.
¿En qué se diferencian los planes de precios?
Ambos planes te permiten acceder por completo a la biblioteca y a todas las funciones de Perlego. Las únicas diferencias son el precio y el período de suscripción: con el plan anual ahorrarás en torno a un 30 % en comparación con 12 meses de un plan mensual.
¿Qué es Perlego?
Somos un servicio de suscripción de libros de texto en línea que te permite acceder a toda una biblioteca en línea por menos de lo que cuesta un libro al mes. Con más de un millón de libros sobre más de 1000 categorías, ¡tenemos todo lo que necesitas! Obtén más información aquí.
¿Perlego ofrece la función de texto a voz?
Busca el símbolo de lectura en voz alta en tu próximo libro para ver si puedes escucharlo. La herramienta de lectura en voz alta lee el texto en voz alta por ti, resaltando el texto a medida que se lee. Puedes pausarla, acelerarla y ralentizarla. Obtén más información aquí.
¿Es Endodontic Microbiology un PDF/ePUB en línea?
Sí, puedes acceder a Endodontic Microbiology de Ashraf F. Fouad, Ashraf F. Fouad en formato PDF o ePUB, así como a otros libros populares de Medicina y Odontotecnica. Tenemos más de un millón de libros disponibles en nuestro catálogo para que explores.
Información
Chapter 1
Microbial Perspectives in the Twenty-First Century
William Wade
1.1 Introduction
The final quarter of the nineteenth century was arguably the golden age of medical microbiology. The ground-breaking work of Pasteur, Koch, and others led to the development of broth and agar media that were able to support the growth in the laboratory of the major bacterial pathogens affecting humans. The ability to grow these organisms in pure culture led to the production of vaccines for many of the diseases they caused. These advances, and the subsequent discovery and development of antimicrobials, led to the mistaken belief that infectious disease had been beaten.
Of course, it is now realized that this optimistic viewpoint is not justified, not least because of the rapid emergence of bacterial resistance to antimicrobials. Indeed, the consensus view is that the battle against bacterial resistance is currently being lost, because of both the difficulty and costs associated with developing new antimicrobials and indiscriminate use of those currently available. The predicted ultimate failure of antimicrobial strategies has led to renewed interest in elucidating the pathogenic mechanisms used by bacteria to cause disease, with the ultimate aim of devising new methods of disease prevention and treatment.
At the same time, interest in the microbial populations of the Earth has been intense and new techniques have become available to characterize the bacterial communities found in every ecosystem on the planet. These have revealed the quite astonishing diversity of microbial life on Earth and the extreme complexity of most bacterial communities. Furthermore, the extent of subspecific diversity is only now being fully appreciated. Bacterial readily exchange DNA and can “shuffle” their own genomes to generate diversity with the ultimate aim of responding and adapting to environmental change. As discussed later, bacteria in communities communicate with each other and, in the case of commensals living with plants and animals, their hosts. These interactions operate at various levels and can be remarkably sophisticated. The twenty-first century will be a period of tremendous advances in our understanding of the microbial world.
The aim of this chapter is to review recent deve- lopments in microbiology and to highlight selected areas that are likely to change our conceptual view of infectious disease as a whole, and oral and endodontic infections in particular. Inevitably, a single short chapter cannot provide a comprehensive overview of an entire discipline, but the interlinked topics covered are those that will undoubtedly change our view of the microbial world and its relationship with the human host.
1.2 Genomics
The sequence of the human genome was published in 2001. The benefits of this outstanding achievement are now being realized with the identification of genes responsible for or causing a predisposition for a large number of diseases (Wellcome Trust Case Control Consortium 2007). At the same time, and largely possible because of the technical advances made as part of the human genome sequencing effort, genomes of other organisms are being sequenced, including those of bacteria.
As of February 2015, the sequencing of the genomes of 26 522 bacteria and 647 archaea had been completed, while 15 800 and 424, respectively, were in progress or available as a draft (for more information see www.genomesonline.org). As expected, the data obtained have revealed the enormous genetic potential contained within bacterial genomes; in each genome sequenced, around one-third of the genes present have been novel and the function of a significant proportion remains unknown.
The availability of genome sequence data is allowing a far more robust bacterial classification to be constructed than previously possible. Bacterial taxonomy was once based purely on phenotypic characters and was very inexact because of the difficulties involved in obtaining and interpreting such data compared to plants and animals where differences in phenotype are far more obvious. In recent years, genetic information has been increasingly used, but on a limited scale, and typically only the sequences of the 16S ribosomal RNA (rRNA) and other housekeeping genes have been used. New methods are now being introduced to make use of the sequence data available for complete genomes (Konstantinidis and Tiedje 2005). In general, the results of using such methods have supported the 16S rRNA gene taxonomy at species and genus level but, in addition, have provided improved clarity of the relationships among the higher taxonomic ranks, where substantial overlap between ranks has been observed.
The results of the analysis of some genomic data have been extremely surprising. A Gram-positive coccus found in amoebae could not be identified by the conventional molecular analysis of 16S rRNA gene sequencing because no ribosomal genes could be amplified for sequencing. Genomic data explained this difficulty by revealing that the organism was actually a virus, the largest yet discovered. Now named Mimivirus, the large virus particles are up to 0.8 μm in diameter, the size of many bacteria. It primarily infects amoebae but has been implicated as a cause of pneumonia on serologic grounds and has caused a laboratory-acquired pneumonia in a researcher (Raoult et al. 2007). At the other end of the bacterial scale, members of the genus Epulopiscium, found in the intestine of certain surgeonfish (Angert et al. 1993), have been discovered that are visible with the naked eye.
In addition to correctly identifying evolutionary oddities, genomic data have identified numerous novel biochemical pathways with the potential for exploitation. Among these are some novel antimicrobials although the range of targets within bacterial cells that has arisen by natural evolution is rather narrow. A more promising avenue to the development of novel antimicrobials is to use genomic data to identify novel targets for antimicrobial treatments (Pucci 2006). Predictions can be made from genome data as to how essential a given gene is to an organism and therefore how disrupting the gene would affect the vitality of the organism. These predictions can then be tested in an appropriate manner experimentally using a wide range of methods that have been developed in response to the availability of genomic data. These include random mutagenesis mediated by transposons or insertion of plasmids, targeted gene disruption or in vivo techniques such as signature-tagged mutagenesis and in vivo expression technology. Structural genomics, where sequence data is used to predict the structure of essential bacterial proteins, is also being used to identify potential targets for antimicrobials. Finally, comparative genomics can be used to identify common features of pathogens affecting a particular body site to custom design antimicrobials for specific purposes, for example, respiratory tract infection.
Next generation sequencing technologies such as the Roche 454 and Illumina systems have been introduced and have brought the ability to sequence bacterial genomes within the reach of individual laboratories. Accurate interpretation of the data remains a challenge, however, although a number of useful software programs are now available (Edwards and Holt 2013). The information obtained thus far has been of extraordinary value in understanding the role of pathogenic bacteria in disease and is the fundamental basis of other new technologies such as transcriptomics and proteomics. The next task will be to understand how gene products interact both within a bacterial cell and in response to external stimuli from the environment and other organisms.
1.3 Molecular microbial ecology and the study of uncultivable bacteria
Almost without exception, oral infections are polymicrobial in nature and difficult to study because around half of the bacteria present in the oral cavity cannot be grown using conventional culture media. It has long been recognized that not all bacteria from a given habitat can be cultured on artificial media in the laboratory. Indeed, it has been estimated that less than 2% of bacteria on Earth can be cultured.
Methods for the characterization of complex bacterial communities were developed as a consequence of the use of DNA sequence data for the construction of evolutionary trees. This was done by comparing the sequences of genes encoding essential functions, the so-called housekeeping genes that are found in all cellular organisms. The gene most commonly used to date has been encoding the small subunit (16S) rRNA molecule. Ribosomes have the essential function of translating messenger RNA (mRNA) into amino acid chains and, because of the need to preserve function, have evolved slowly. Some of the regions of the gene have changed very little over time and are therefore virtually identical in all bacteria. These regions are very useful for the design of universal polymerase chain reaction (PCR) primers that can amplify the gene from a wide range of different bacteria. Other regions are more variable and can be used to discriminate between organisms, almost to species level. Woese and colleagues used small subunit rRNA comparisons to construct a tree of life (Figure 1.1), which showed that bacteria had evolved into two domains, the Archaea and Bacteria, while eukaryotic organisms fell into a single third domain, the Eukarya (Woese 1987). It was originally thought that organisms found in the domain Bacteria were those found in normal environments while the Archaea were present in extreme environments such as the deep sea and associated with volcanoes and so on. However, these associations have since been shown not to be true and members of Archaea are now known to be widely distributed, and an archaeal genus, Methanobrevibacter, can be found in the human mouth.
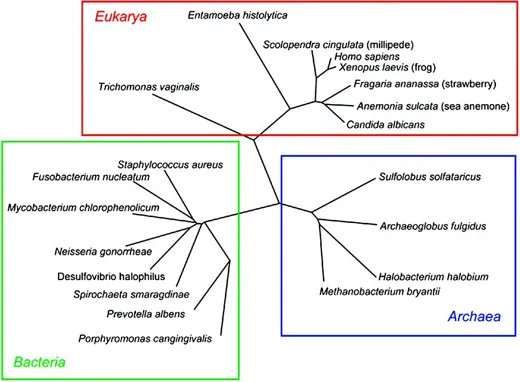
Fig. 1.1 Phylogenetic tree showing representatives of the domains Eukarya, Archaea, and Bacteria.
A major consequence of the availability of this tree is that unknown organisms of any type can be identified simply by sequencing their rRNA gene and adding the sequence to the tree or by directly comparing the sequence with the hundreds of thousands of bacterial sequences held in the sequence databases. Complex bacterial communities can be characterized by the PCR, cloning, and sequencing of 16S rRNA. Such studies have been performed with samples from the human mouth in health and disease and are described in more detail in Chapter 5 and throughout the textbook. A common finding of every study to date has been to confirm that around half of the oral microbiota is uncultivable. Around 700 species have been detected, 95% of which belong to the phyla Firmicutes, Bacteroidetes, Proteobacteria, Actinobacteria, Fusobacteria, and Spirochaetes (Dewhirst et al. 2010). Other phyla consistently detected are Synergistetes, Chloroflexi, and the un-named phylum-level divisions GN02, SR1, and TM7 (Camanocha and Dewhirst 2014). The Human Oral Microbiome Database (www.homd.org) lists the bacterial taxa found in the mouth and provides descriptions of their phenotypes, where available, with links to genome sequence data as well as a 16S rRNA gene sequ...