![]()
PART 1
PLASTICS AS A DESIGN MATERIAL
Plastics materials have a number of unique properties that allow a wide variety of solutions to many design problems. The nature of some of these properties requires the designer to approach the application of these materials to a product design a little differently than many traditional design materials. This section will review common materials and processes and look at some of the general engineering approaches that need to be taken in developing a plastic product design.
![]()
Chapter 1
Introduction to Plastics Materials
In this chapter we will briefly discuss the history of plastics, examine what plastics are, how they are made and some of the general properties of plastics materials. We will also look at the overall size of the plastics industry today.
1.1 History of Plastics
It is hard to imagine a world without plastics, but plastics are a family of relatively new materials and have been around for a little more than 100 years. The start of the plastics industry dates back to 1868 when John Wesley Hyatt, in search of an alternate material to ivory for billiard balls, discovered celluloid, the first commercially successful plastic material. Celluloid also found application in photographic still and movie film and shirt collars and buttons. It is still in use today to make ping pong balls.
Celluloid was a modified naturally occurring polymer, cellulose. In 1907 Dr. Leo Baekeland, through a condensation reaction of phenol and formaldehyde, invented phenolic, the first plastic produced entirely from synthetic materials. This was an easily moldable, cost effective material that became widely used in electrical components and general moldings. Its major limitation was that it was only available in dark colors. This problem was solved in 1929 when American Cyanamid Company introduced urea formaldehyde thermoset molding compounds which could be produced in a wide array of colors.
In 1934 Dr. Wallace Carothers, working for DuPont, invented nylon. This is notable because he was hired to develop a synthetic material to replace silk and he developed a polymer to meet this specific need, a first for polymer chemists.
The first inorganic polymer, polytetrafluoroethylene, more commonly known as Teflon®, was discovered by another DuPont chemist, Dr. Roy Plunkett, in 1938.
Throughout the 1940s thermoset materials dominated the plastics market, but starting in the 1950s new thermoplastic materials and processes began to take over. The first commercial reciprocating screw injection molding machine appeared in Germany in the mid-1950s from Ankerwerk. Due to its ability to produce significantly improved thermoplastic melts, numerous manufacturers around the world soon offered their own versions. Injection-molded thermoplastics started to replace many thermoset applications and many new opportunities for growth were found.
In 1953, the first reinforced plastic car bodies appeared in the Chevrolet Corvette [1], and plastics continue to make inroads in the auto industry as their low costs and high strength-to-weight ratios help engineers meet ever-increasing fuel economy requirements. Use of plastics materials to reduce the weight of cars is a major strategy of the automobile industry as they strive to meet the US National Highway Traffic Safety Administration 2025 CAFE (Corporate Average Fuel Economy) standards of 54.5 miles per gallon by 2025.
Advances in polymer chemistry and catalysts now allow polymer chemists to scientifically develop plastic materials with specific properties to meet the needs of specific applications. Stereospecific catalysts like Zeigler Natta catalysts and metallocene catalysts can help control how and where the molecules attach to one another. Ruthenium catalysts enable ring opening metathesis polymerization, which has opened the possibilities of new families of high performance polymers.
This is allowing plastics to move into areas of much more demanding functional requirements and to be used in a wide array of engineering applications. Plastic materials are not only used in housewares, toys and packaging, but also aerospace, construction, electronics, transportation and industrial applications.
1.2 Definition of Plastics
What actually is a plastic material? There are many similar definitions used, but for our purposes, plastics are materials that are composed of large molecules that are synthetically made and, under the proper conditions, can be readily formed or molded into the desired shape. The large plastic molecules are called polymers from the Greek words poly, which means many, and meros which means units. The polymer is made up of many smaller molecules called monomers which are joined together through chemical bonding, generally through either a condensation or an addition polymerization reaction. The chemical properties of the monomer will determine if and how it can form into a polymer, as well as what properties the finished polymer might have.
1.3 Thermoplastics and Thermosets
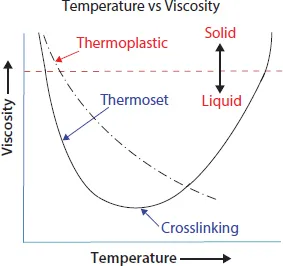
Plastics are divided into two basic families, thermoplastics and thermosets. Thermoplastics are materials that when heated will soften and flow, allowing the polymer chains to slide over one another, and when cooled, they will harden. This process can be repeated many times. This allows thermoplastic materials to be easily recycled and reused. A thermoset material will soften and flow when it is heated, but additional heat will cause a chemical reaction called crosslinking to occur. In crosslinking, chemical bonds form between the polymer chains. This crosslinking reaction locks the polymer chains together and prevents them from sliding over one another, causing the polymer to harden. This process is irreversible. As a result, parts made from thermosets cannot be easily recycled.
Figure 1.1 shows the differences in how thermoplastics and thermosets respond to changes in temperature. At lower temperatures (upper left on the chart) both thermoplastics and thermosets are solids (usually – although a thermoset resin can start out as a liquid). As the temperature is increased, the viscosity (resistance to flow) of both materials will lower until they go from a solid state to a viscous (thick) liquid. As the temperatures continue to increase, the viscosity of both materials continues to decrease. With thermoplastics, this drop in viscosity continues as temperatures increase (up until the chemical bonds start to disassociate and the polymer begins to degrade). With thermosets, the material will rapidly drop in viscosity with increasing temperature until chemical crosslinks start to form between the chains. At that point, viscosity will increase as the crosslinks restrict the polymer chains from sliding past one another. With thermoplastics, we can drop the temperature and reverse the process. With thermosets, once the crosslinks start to form, we cannot reverse the process and the material is said to be cured.
1.4 How Plastics are Made
Polymers are generally formed by two different mechanisms, addition polymerization and condensation polymerization. In addition polymerization the monomer is introduced into the reactor and a chemical initiator is added to create a free radical. The free radical will bond with another monomer, creating a new free radical at the same time. This process will repeat, causing the polymer chain to continue to grow. The growth process will continue until the monomer supply is exhausted, two free radicals meet, or a quenching molecule(s) is added to stop the reaction. Addition polymerization processes can be scaled up to a very large size, has no byproducts (the initiator is consumed by the reaction) and are very economical. Addition polymerization is also called chain-growth polymerization. The common high volume commodity plastics (see Chapter 3) are manufactured by this method. In condensation polymerization two different monomers are introduced into the reactor vessel. One end of molecule 1 will react with one end of molecule 2 and usually as part of this process a water molecule is also created (hence the name “condensation polymerization”). Newly formed molecule 1–2 reacts with either a molecule 1 or a molecule 2, extending the polymer chain. The reaction is ended by either adding a quenching molecule or cooling the process down to stop the reaction. Usually, catalysts are added to help drive and control the reaction. Unlike initiators, catalysts are not consumed by the reaction and must be removed from the finished polymer, along with the water that was produced in the reaction. Many of the engineering polymers are made by condensation polymerization. This is generally a more expensive process and will increase the cost of plastics made by this method. Condensation polymerization is also called stepwise polymerization.
1.5 General Plastics Properties
There are a large number of commercial polymers available, offering a wide range of properties that can be used in a very diverse set of applications. In general, plastics offer a low density resulting in a low specific weight (weight per unit volume) giving a high strength to weight ratio over competing materials. This characteristic is finding value for weight reduction in transportation applications, such as automobiles, aircraft and high-speed trains, to improve fuel economy. Plastics typically have low melting points which help processing but limit applications in high temperature environments. Although plastics do have lower melting temperatures than many competing materials, there are plastics that can withstand some surprisingly high temperatures, allowing them to be used in under the hood automobile applications and for sterilizable parts. They have a low thermal conductivity which makes them ideal for many thermal insulation applications. They also have good electrical insulative characteristics which make them key materials in a wide range of electrical applications, including wire and cable insulation, tool housings, circuit boards and components and household appliances. Plastics are available with optical clarity and can be made in an infinite array of colors. Lastly, one of the most significant general properties of plastics is that of relatively low cost, which has led plastics to be used in a number of high volume, low cost...