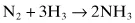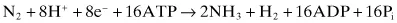![]()
1
Nitrogen Metabolism
Some microorganisms are capable of reducing nitrogen gas to ammonium, which can then be incorporated into amino acids, and thence into other organic nitrogenous compounds, including purines, pyrimidines, amino sugars, phospholipid bases and a variety of cofactors and coenzymes that are vitamins for animals. Plants and other microorganisms can incorporate ammonium and inorganic nitrates and nitrites into amino acids and other nitrogenous compounds. Animals cannot utilize inorganic nitrogen compounds to any significant extent, but rather are reliant on plant foods (and also, to some extent, microorganisms) for amino acids for the synthesis of tissue proteins and other nitrogenous compounds, including purines and pyrimidines. Other organic nitrogenous compounds in plant foods can be utilized to a greater or lesser extent.
Ruminants are able to make use of inorganic nitrogen compounds indirectly, because of their large intestinal population of commensal bacteria that can synthesize amino acids from ammonium. This is economically important, since chemically synthesized urea fed to ruminants releases more expensive protein-rich oil-seed cake and protein from bacteria, yeasts and fungi for human consumption, or as feedstuff for monogastric livestock.
The major end products of amino acid catabolism by animals are relatively simple organic compounds such as urea, purines and uric acid, as well as ammonium salts (and in some cases ammonia gas) and nitrate and nitrite salts. Various microorganisms can oxidize ammonia to nitrogen gas, reduce nitrites and nitrates to nitrogen gas or catalyze a reaction between ammonia and nitrite to produce nitrogen gas.
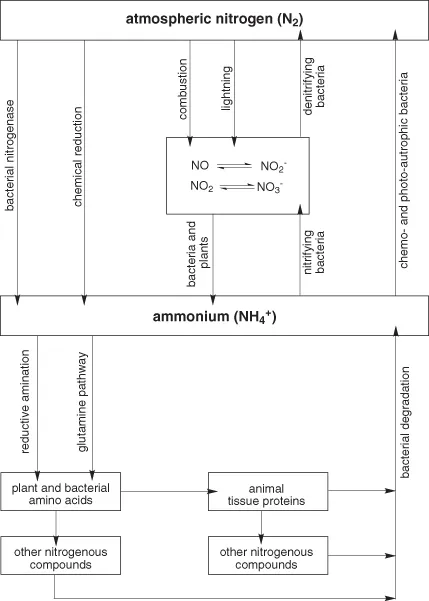
There is, thus, a cycle of nitrogen metabolism:
- nitrogen gas is fixed as ammonium;
- ammonium is incorporated into amino acids;
- other nitrogenous compounds are synthesized from amino acids;
- this is followed by catabolism, ultimately yielding ammonium and nitrates, then denitrification reactions releasing nitrogen gas.
This nitrogen cycle is shown in Figure 1.1.
As a result of human activity, the nitrogen cycle is no longer in balance. There is an excess of nitrogen fixation overdenitrification, resulting in the accumulation of fixed nitrogen in rivers, lakes and oceans and of nitrogen oxides in the atmosphere. Global production of nitrogen fertilizers was 80 × 106 million tonnes in 1997, and is projected to rise to 134 × 106 million tonnes by 2020; half of all the chemically synthesized nitrogen fertilizer used up until 1990 was used between 1980 and 1990.
The burning of fossil fuels and biomass accounts for release into the atmosphere of some 20 × 106 tonnes of nitrogen oxides each year, and lightning probably produces about half as much. It is estimated that terrestrial ecosystems produced 90–140 × 106 tonnes of fixed nitrogen a year prior to human activity and that widespread cultivation of legume crops has added 32–55 × 106 tonnes of fixed nitrogen per year. Marine ecosystems are estimated to fix 30–300 × 106 tonnes of nitrogen a year. Overall, human activities are estimated to fix 210 × 106 tonnes of nitrogen a year, compared with 140 × 106 tonnes from biological nitrogen fixation and the action of lightning (Galloway et al., 1995; Vitousek et al., 1997).
There are two consequences of this excess of nitrogen fixation overdenitrification. Nitrous oxide (N2O) is a greenhouse gas, and hence it contributes to global warming and climate change. It also catalyzes the destruction of ozone in the stratosphere. Nitrates in drinking water present a health hazard; gastric microorganisms reduce nitrate (NO3−) to nitrite (NO2−), which can react with haemoglobin to yield methaemoglobin, which does not transport oxygen. Although mammals have methaemoglobin reductase and can regenerate active haemoglobin, young infants are especially at risk from excessive nitrate intake, because foetal haemoglobin is considerably more sensitive to nitrite than is adult haemoglobin.
A nitrate concentration greater than 10 mg N/l of water is considered to pose a threat to public health. Nitrites are also able to react with amines under the acidic conditions of the stomach to form carcinogenic nitrosamines, although it is not clear whether the small amounts of nitrosamines formed from dietary amines and nitrites pose a significant health hazard. There is therefore great interest in bacteria that can be used to denitrify drinking water (section 1.2; Martinez-Espinosa et al., 2011).
1.1 Nitrogen Fixation
The N ≡ N triple bond in nitrogen gas is extremely stable, with a bond energy of 0.94 MJ (225 kcal) per mol; this is the bond that has to be broken to fix nitrogen. The Haber-Bosch process for synthesis of ammonia (the basis of the chemical fertilizer industry) uses temperatures of 300–550°C and pressures of 15–25 MPa (150–250 atm), with an iron catalyst, to reduce nitrogen with hydrogen gas to form ammonia:
Nitrogen-fixing microorganisms (diazotrophes) catalyze the same reaction at temperatures as low as 10°C and 100 kPa (1 atm) pressure. This bacterial nitrogen fixation accounts for some 100 × 106 tonnes of nitrogen per year. As shown in Table 1.1, the bacteria and cyanobacteria (formerly known as blue-green algae) that catalyze nitrogen fixation occupy a wide variety of ecological niches. Among heterotrophic bacteria, diazotrophes may be obligate or facultative anaerobes or obligate aerobes, and autotrophic diazotrophes may be aerobic or anaerobic, photosynthetic or non-photosynthetic. Non-photosynthetic autotrophic diazotrophes include those that can reduce sulphate to sulphide (e.g. Desulphovibrio spp.) and the methanogenic archaea.
Table 1.1 Some organisms capable of fixing nitrogen.
| Free-living heterotrophes | obligatory aerobic | Azotobacter spp., |
| Mycobacterium spp. |
| facultatively anaerobic | Klebsiella pneumoniae, |
| Bacillus polymyxa |
| obligatory anaerobic | Clostridium pasteurianum, |
| Clostridium butyricum |
| Free-living autotrophes | obligatory aerobic | cyanobacteria: |
| Anabaena spp., Nostoc spp., |
| Plectonema spp. |
| facultatively anaerobic | Rhodospirillum spp., Rhodopseudomonas spp. |
| obligatory anaerobic | Chromatium spp., |
| Chlorobium spp. |
| Symbiotic associations | fungi (lichens), liverworts, tropical grasses, Azolla spp. | cyanobacteria |
| plant leaf nodules | Klebsiella spp. |
| roots and leaves of plants | Azotobacter spp. |
| legume root nodules | Rhizobium spp. |
| non-legume root nodules | Frankia spp. |
Although the ability to fix nitrogen is found in bacteria and archaea occupying a wide variety of ecological niches, only a few hundred prokaryotic species (and no eukaryotes) are diazotrophic. Free-living heterotrophic bacteria have proven to be the easiest organisms in which to study nitrogen fixation, but they make a relatively minor contribution to global nitrogen fixation compared with photoautotrophic and symbiotic organisms.
A number of plant-bacteroid symbiont pairs are also diazotrophic. The best known is the symbiotic association of Rhizobium spp. in root nodules of legumes (section 1.1.1.7), but a number of other diazotrophic organisms (e.g. Frankia spp.) form symbiotic associations with non-leguminous plants. Rhizobium and Frankia are obligate symbionts, and are not capable of independent existence. A number of organisms that are both capable of independent existence and capable of fixing nitrogen when free-living, such as Azotobacter spp. and cyanobacteria, frequently form symbiotic associations in leaf nodules of higher plants or around the roots of aquatic plants. Many lichens, which are symbionts of fungi with bacteria or cyanobacteria, are diazotrophic.
Some nitrogen-fixing endophytic bacteria form nodule-independent associations with cereal crops, but it is unclear whether the effect on plant growth is due to nitrogen fixation or to the synthesis of bacterial metabolites that act as plant growth hormones by the bacteria.
A major challenge for plant science is the possibility of engineering nitrogen fixation into non-leguminous crops. There are two possible approaches to this (Beatty & Good, 2011). It may be possible to transfer nitrogen-fixing genes directly into cereal crops and ensure their expression in the roots (section 1.1.1.1), or it may be possible to bio-engineer cereal crops to produce the same chemo-attractants for nitrogen-fixing bacteria as are produced by legumes (section 1.1.1.7).
Some wood-eating insects (e.g. termites) and molluscs (e.g. the shipworm, Teredo spp.) have symbiotic diazotrophic bacteria that may make a significant contribution to the host’s nitrogen nutrition. Commensal bacteria in ruminants fix nitrogen, but there is no evidence that non-ruminant mammals (including human beings) harbour any significant number of intestinal nitrogen-fixing bacteria.
There are three requirements for nitrogen fixation: the enzyme nitrogenase, which catalyzes the reduction of N2 to NH4+; a source of reductant; and an electron carrier to couple the reductant with the enzyme. In addition, there is a requirement for 16 × ATP per mol of nitrogen reduced to ammonium. In Clostridium spp. as much as 30 per cent of the metabolic energy derived from anaerobic fermentation may be utilized in nitrogen fixation.
1.1.1 Nitrogenase
There are three related families of proteins that catalyze the reduction of nitrogen gas to ammonia. The most studied contains both molybdenum and iron, but there are also nitrogenases that contain vanadium instead of molybdenum, and some that contain only iron. These different nitrogenases are encoded by different genes and, in some microorganisms, all three enzymes are expressed. There is considerable sequence homology between the different nitrogenases and also between the same types of nitrogenase (Mo-Fe, V-Fe and Fe) from different organisms. Nitrogenases may utilize either ferredoxin or flavodoxin as the reductant (Eady, 1996; Howard & Rees, 1996).
The reaction catalyzed by nitrogenase is:
Two separate proteins make up nitrogenase: an iron-containing protein that is a homodimer with two ATP binding sites and a single iron-sulphur cluster (4Fe4S) shared between the two subunits; and the iron-molybdenum protein, which is a hetero-tetramer (2α2β) with two iron-sulphur clusters (8Fe7S) and two mol of the molybdenum coenzyme (7Fe-Mo-9S-homocitrate). The two αβ subunits of this protein seem to be independent; both catalyze the reduction of nitrogen, so that the tetramer has two catalytic sites.
The main function of the iron protein is to transfer reducing equivalents to the molybdenum-iron protein. It is sometimes called nitrogenase reductase, but it is also required for the synthesis of the iron-molybdenum cofactor and its insertion into the iron-molybdenum protein. Each of the eight electron transfer reactions required for the reduction of 1 mol of nitrogen involves association between the iron protein and the iron-molybdenum protein, then dissociation of the complex (Burgess & Lowe, 1996; Howard & Rees, 1996; Rubio & Ludden, 2008).
In the reduced iron protein, the (4Fe4S) cluster is in the +1 oxidation state, and the prote...