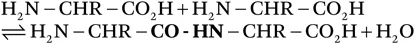Tanning Chemistry
The Science of Leather
Anthony D Covington, William R Wise
- 660 pagine
- English
- ePUB (disponibile sull'app)
- Disponibile su iOS e Android
Tanning Chemistry
The Science of Leather
Anthony D Covington, William R Wise
Informazioni sul libro
This book offers a state-of-the-art view of leather making, based on the scientific principles underpinning the technology. In particular, it contributes to the understanding of the modern leather industry, allowing practitioners to make judgements about day-to-day problems in the tannery and how change can be applied in a predictable way. Major themes running through the book are the economics and environmental impact of leather making and how these will ensure the sustainability of the industry.
This second edition of Tony Covington's Tanning Chemistry is a revision, update and extension in collaboration with a new co-author, Will Wise. The update reflects the advances made in the past decade, including a discussion of the impact of new information concerning the chemistry of sulfide. The original chapters have been re-organised and new chapters on novel modes of reagent delivery and the principles of finishing are now included. Enzymology is addressed as a separate topic, as are environmental impact and the future of leather.
The book will be useful to all those involved in the supply chain, from farm, through students, chemical suppliers and tanners, to leather goods brands. Leather science is the key to understanding leather technology, to make it work, to make it work better and to keep it ahead of the competition.
Domande frequenti
Informazioni
In order to understand the principles that underpin the conversion of hide or skin to leather, it is necessary to know the fundamental structure of the raw material and how that structure might be modified chemically. The chemistry of collagen defines not only the sequencing of its amino acid constituents but also the physical nature of its structure and how it creates levels of structure or a hierarchy. This depends on the chains creating a triple helix as the basic unit of structure. The chemical properties of collagen are defined by the sidechains on the helices, which may be charged, depending on the pH; in this way, collagen can undergo a wide range of covalent or electrostatic reactions, which are the basis for tanning processes. At the heart of the chemistry of collagen is the relationship with water, which is an integral feature of structure: the supramolecular matrix of water around the triple helices provides the region for chemical modification, leading to tanning technology.
1.1 Introduction
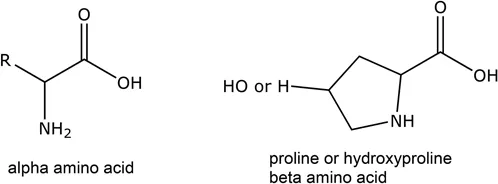
| Name | Abbreviation | Type | Sidechain: R = | Importance in leather making |
| Glycine | Gly | α, neutral | –H | Collagen structure |
| Alanine | Ala | α, neutral | –CH3 | Hydrophobic bonding |
| Valine | Val | α, neutral | –CH(CH3)2 | Hydrophobic bonding |
| Leucine | Leu | α, neutral | –CH2CH(CH3)2 | Hydrophobic bonding |
| Isoleucine | Ileu | α, neutral | CH3CH2CH(CH3) | Hydrophobic bonding |
| Phenylalanine | Phe | α, neutral | –CH2C6H5 | Hydrophobic bonding |
| Serine | Ser | α, neutral | –CH2OH | Unhairing |
| Cysteine | CySH | α, neutral, S containing | –CH2SH | Unhairing |
| Cystine | CyS–SCy | α, neutral, S containing | –CH2SSCH2– | Unhairing |
| Aspartic acid | Asp | α, acidic | –CH2CO2H | Isoelectric point (IEP),a mineral tanning |
| Asparagine | Asn | α, neutral | –CH2CONH2 | IEP |
| Glutamic acid | Glu | α, acidic | –(CH2)2CO2H | IEP, mineral tanning |
| Glutamine | Gln | α, neutral | –(CH2)2CONH2 | IEP |
| Arginine | Arg | α, basic | –(CH2)3NHC(NH)NH2 | IEP |
| Lysine | Lys | α, basic | –(CH2)4NH2 | IEP, aldehydic tanning, dyeing, lubrication |
| Histidine | His | α, basic | 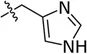 | Aldehydic tanning, dyeing, lubrication |
| Proline | Pro | β, neutral | See Figure 1.1 | Collagen structure |
| Hydroxyproline | Hypro | β, neutral | See Figure 1.1 | Collagen structure, hydrogen bonding |